Live and let die
Protein prevents immune cell suicide
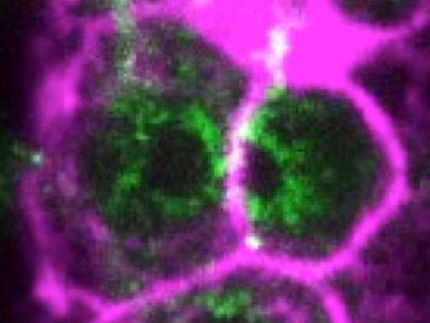
A protein called c-FLIP-R is critical to immune cell survival: If this molecule is missing, the cells kill themselves – and are thus no longer able to perform their job fighting off invaders.
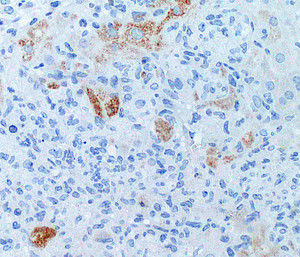
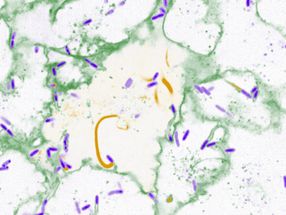
Liver cells under the microscope. Apoptotic cells are stained in brown, cell nuclei are blue.
© HZI / Schmitz
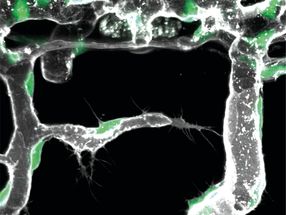
Apoptosis, programmed cell death, is a kind of cellular suicide program. If something triggers it, the cells perish in a controlled manner. A certain type of white blood cell called a lymphocyte commits suicide by apoptosis, which is one way for the body to effectively shut down an immune response once a pathogen has been successfully fought off. To make sure this doesn’t happen until after the job is done, apoptosis is a tightly regulated process. If this regulation is lost, potential consequences include autoimmune diseases, neurodegenerative diseases, even different forms of cancer.
The protein c-FLIP figures prominently into how the body regulates this process. The protein exists as three different versions, or isoforms. While the roles of c-FLIP-L and c-FLIP-S isoforms are well established, until now relatively little has been known about the role of the c-FLIP-R isoform. Which is why Prof. Ingo Schmitz and the Braunschweig team of researchers decided to take a closer look at this particular isoform. What they found is that it confers protection on immune cells against the apoptosis program. “If c-FLIP-R is activated, lymphocytes are apoptosis-resistant,” says the head of the HZI’s Systems-oriented Immunology and Inflammation Research work group, who also teaches at the OvGU. “Apoptosis can take place only in the protein’s absence.”
Traditionally, insights like these are based on research on mice and have yet to be confirmed in humans. In this case, however, the exact opposite holds true: Schmitz and his team initially made their observations on human blood cells. Next, to test these findings at the organismal level, the researchers examined a certain strain of mice that were making c-FLIP in all of their blood cells. The result: “Although the animals’ immune system was unchanged, c-FLIP prevents their white blood cells from undergoing apoptosis,” explains immunologist Prof. Dunja Bruder, who also teaches in Magdeburg.
As a result, the mice are less susceptible to Listeria infections. The bacterium, which also infects humans, is transmitted via contaminated foods. “The mice have a lower bacterial count and a lesser extent of organ damage than is normally the case with Listeria,” says Bruder. Consistently high levels of c-FLIP, however, would permanently disable apoptosis in activated lymphocytes and could trigger different autoimmune diseases. The scientists are hoping to conduct further, more in-depth research on this particular topic.
Still, for Ingo Schmitz, there is one obvious potential medical benefit to the discovery: “If we were able to simply turn on c-FLIP-R, this would help strengthen the immune system.” Helping the body heal itself if you will. A highly promising approach that could prove important to pretty much every single disease. Schmitz is already working on mapping out the search for candidate active substances.
Original publication
Other news from the department science

Get the life science industry in your inbox
From now on, don't miss a thing: Our newsletter for biotechnology, pharma and life sciences brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.