Jumping gene could provide non-viral alternative for gene therapy
Advertisement
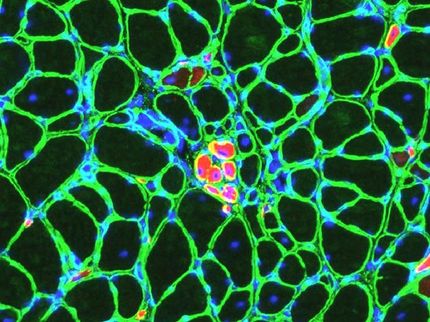
A jumping gene first identified in a cabbage-eating moth may one day provide a safer, target-specific alternative to viruses for gene therapy, researchers say. They compared the ability of the four best-characterized jumping genes, or transposons, to insert themselves into a cell's DNA and produce a desired change, such as making the cell resistant to damage from radiation therapy. They found the piggyBac transposon was five to 10 times better than the other circular pieces of DNA at making a home and a difference in several mammalian cell lines, including three human ones.
"If we want to add a therapeutic gene, we can put it within the transposon and use it to deliver the gene into the cell," says Dr. Joseph M. Kaminski, radiation oncologist at the Medical College of Georgia Cancer Center and a corresponding author on research published in Proceedings of the National Academy of Sciences Early Edition. "You can use these wherever retroviruses have been used."
In addition to piggyBac, researchers looked at what was believed to be the most efficient transposon in mammalian cells, hyperactive Sleeping Beauty, first found "asleep" in fish. They also looked at Tol2, another fish transposon, and Mos1, found in insects. The piggyBac transposon, which has close relatives in the human genome, is widely used to genetically modify insects. Sleeping Beauty has been used to correct hereditary diseases, including hemophilia, in a mouse model.
For this study, researchers used transposons to deliver an antibiotic-resistant gene. "It's a way of screening and seeing which transposon is better," Dr. Kaminski says. They found that while piggyBac might not work as efficiently as a virus, it put Sleeping Beauty to shame when it came to making cells antibiotic-resistant.
"Sleeping Beauty has captured the field as far as transposons to be used in mammals," says Dr. Stefan Moisyadi, molecular biologist, at the University of Hawaii and a corresponding author. "But by comparing different transposons, we showed Sleeping Beauty is far inferior to piggyBac."
Dr. Kaminski's previous work hypothesized that the integration site for transposons can be selected. "Typically, viruses and transposons will integrate anywhere along the genome," he says. "If they integrate anywhere, it can obviously cause harm. If we can target the integration, be able to insert the gene at a safe spot in the genome, that would be beneficial." He confirmed that targeting integration is possible in a paper he co-authored in 2005 also in The FASEB Journal. "We can do it in insects," says Dr. Moisyadi. "I think it's a short step to take it to a targeting mechanism we can use in humans."
Another clear benefit is that transposons are cheaper to produce and probably safer than viruses. For example, retroviruses use RNA to make DNA, an error-prone process that must occur before integration, Dr. Kaminski says. Also, viruses can't carry larger genes, such as the dystrophin gene, which could help correct muscular dystrophy. On the other hand, unlike retroviruses, transposons have to be coated with lipid to slip into cells.
Although piggyBac is not as successful as the virus at integrating into DNA, "we could potentially make a hyperactive version of piggyBac, like they did for Sleeping Beauty, which might be as good or better than retroviruses," Dr. Kaminski says. "I think we'll do it or somebody will. I think it's a safer method."
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Gene therapy
Genetic diseases once considered untreatable are now at the center of innovative therapeutic approaches. Research and development of gene therapies in biotech and pharma aim to directly correct or replace defective or missing genes to combat disease at the molecular level. This revolutionary approach promises not only to treat symptoms, but to eliminate the cause of the disease itself.

Topic world Gene therapy
Genetic diseases once considered untreatable are now at the center of innovative therapeutic approaches. Research and development of gene therapies in biotech and pharma aim to directly correct or replace defective or missing genes to combat disease at the molecular level. This revolutionary approach promises not only to treat symptoms, but to eliminate the cause of the disease itself.