Cell Genesys Reports Clinical Data From GVAX® Vaccine For Lung Cancer Program
Advertisement
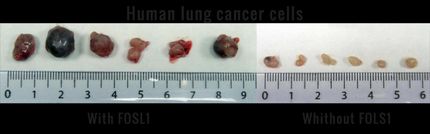
Cell Genesys, Inc. reported data from a Phase 2 clinical trial of GVAX® vaccine for lung cancer, a patient-specific vaccine for patients with advanced-stage, non small-cell lung cancer (NSCLC). The trial, which is the second Phase 2 trial in this indication, enrolled 101 patients who were randomized to receive vaccine with or without low-dose cyclophosphamide, a chemotherapeutic agent that has been shown to enhance immune response at low doses. Vaccine was prepared from the patient's own tumor obtained through various surgical procedures. Available data from 52 patients who have initiated vaccine treatment showed that one patient with adenocarcinoma of the lung had a near complete response and remains progression free for more than 16 months after treatment. Fourteen other patients had stable disease. Median overall survival was 5.4 months for the vaccine-only treatment arm and 9.5 months for the cyclophosphamide-plus-vaccine treatment arm, a difference that was not statistically significant. The administration of GVAX® vaccine for lung cancer was generally well tolerated. The findings from this clinical trial were presented today at the 11th World Conference on Lung Cancer meeting held in Barcelona, Spain, by Dr. Joan Schiller from the University of Wisconsin.
Cell Genesys recently announced that it will discontinue further clinical development of GVAX® vaccine for lung cancer in order to focus its resources on the development of its non patient-specific vaccine products for prostate cancer, leukemia and pancreatic cancer. While promising early results have been reported for GVAX® vaccine for lung cancer, new effective therapies for lung cancer have recently been approved which are relatively easy to manufacture compared to patient-specific vaccine products. The company believes that patient-specific vaccine products such as GVAX® vaccine for lung cancer will therefore face a more challenging development and commercialization path.
Clinical trials of GVAX® cancer vaccines are under way for multiple types of cancer including prostate cancer, leukemia and pancreatic cancer. GVAX® vaccines are whole-cell vaccines that are designed to stimulate an immune response against the patient's tumor. The vaccines are comprised of tumor cells that have been genetically modified to secrete GM-CSF, an immune stimulatory hormone that plays a key role in stimulating the body's immune response to vaccines and are being developed as non patient-specific "off-the-shelf" pharmaceutical products. GVAX® cancer vaccines have demonstrated a favorable side effect profile in over 600 patients treated in clinical trials to date.