New Insights into Protein Folding Inside Cells
Chaperone Complex Captured in Action
Advertisement
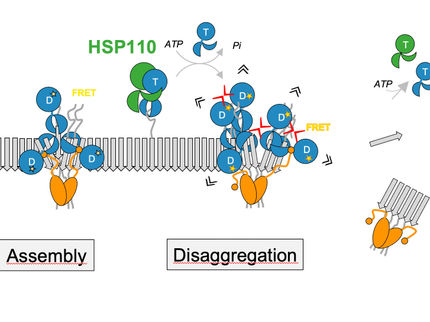
How proteins in our cells attain their correct three-dimensional structure is crucial to their function—and to our health. Errors in this process can lead to serious diseases. Researchers at the Center for Medical Biotechnology (ZMB) at the University of Duisburg-Essen, together with national partners, have now deciphered a central mechanism of this essential cellular process. At the core of their findings is the BiP–GRP94 chaperone complex. It plays a key role in protein folding in the endoplasmic reticulum, the cell's production and control center.. The study has just been published in the journal Nature Structural & Molecular Biology.
“Our study is the first to reveal at the structural level how the chaperones BiP and GRP94 work together,” explains Prof. Dr. Doris Hellerschmied (University of Duisburg-Essen), senior author of the publication. “We were able to show that the complex changes step by step and in a coordinated manner as it performs its function. The conformational plasticity of the BiP–GRP94 complex is likely key to its ability to recognize and process a wide variety of protein folding states.”
Using cutting-edge techniques such as high-resolution electron microscopy and biochemical analyses, the researchers visualized several previously unknown conformations of the chaperone complex and decoded their functional significance.
“Our results provide valuable insights into the cell’s molecular machinery,” says Dr. Simon Pöpsel (University of Duisburg-Essen), co-lead author of the study. “In the long term, this knowledge could contribute to the development of new therapeutic approaches for diseases in which protein folding is disrupted—such as certain neurodegenerative disorders.”
Original publication
Joel Cyrille Brenner, Linda Charlotte Zirden, Lana Buzuk, Yasser Almeida-Hernandez, Lea Radzuweit, Joao Diamantino, Farnusch Kaschani, Markus Kaiser, Elsa Sanchez-Garcia, Simon Poepsel, Doris Hellerschmied; "Conformational plasticity of a BiP–GRP94 chaperone complex"; Nature Structural & Molecular Biology, 2025-7-14