Anti-fibrotic peptide shows early promise against interstitial lung disease
Advertisement
The results of preclinical studies by investigators at the Medical University of South Carolina (MUSC) suggest that the M10 peptide could help protect against fibrotic damage in patients with systemic sclerosis, particularly in those who develop interstitial lung diseases (ILD), its deadliest complication.
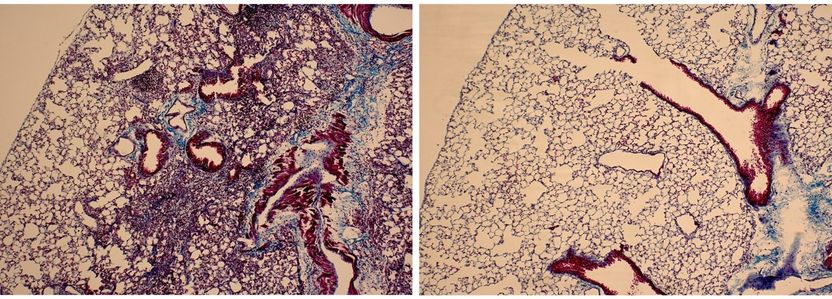
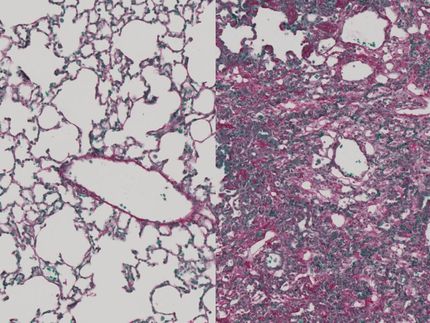
Lung tissue isolated from mice that received bleomycin is characterized by extensive infiltration of inflammatory cells, thickening of the alveolar walls, and multiple fibrotic lesions with excessive amounts of extracellular matrix proteins (left). Lung tissue from mice that received bleomycin and M10 treatment demonstrates significant reduction of cellular infiltrates, decreased thickness of alveolar septa, and reduced accumulation of extracellular matrix proteins (right).
Reproduced from Translational Research (http://www.translationalres.com), Volume 170, April 2016, Pages 99-111, with permission from Elsevier.
Fibrotic diseases, which are characterized by excessive scarring due to overproduction by fibroblasts of collagen or extracellular matrix, account for more than 45% of U.S. deaths--more than cancer--and are estimated to cost $10 billion annually. Despite the prevalence of fibrotic diseases, only a handful of anti-fibrotic agents have been approved by the U.S. Food and Drug Administration, and none is available for systemic sclerosis.
In many ways, systemic sclerosis is the quintessential fibrotic disease, since its scarring can damage any part of the body. "Systemic sclerosis is often more than skin deep, affecting the gastrointestinal tract, the lungs, the heart, the kidneys, and the blood vessels, so it is a model for many other more prevalent fibrotic diseases," says Richard M. Silver, M.D., Director of the Division of Rheumatology and Immunology at MUSC and a co-author on the article. "Whereas there may be 300,000 Americans with scleroderma/systemic sclerosis, millions of others suffer from fibrosis of these other organ systems."
M10 is a ten-amino acid peptide formed from the natural cleavage of the receptor tyrosine kinase MET by the caspase 3. MET, also known as hepatocyte growth factor receptor, is thought to protect against injury and fibrosis, but the mechanisms by which it does so have remained unclear.
The MUSC investigators showed that M10 could protect against fibrotic injury in a bleomycin-induced model of ILD and that its anti-fibrotic effects are likely due to its modulation of the transforming growth factor beta 1 (TGF-β1) pathway. TGF-β1 is a cytokine that has been implicated in inflammation and fibrosis.
"We observed that treatment with M10 by intraperitoneal injection markedly improved bleomycin-induced lung fibrosis in mice, suggesting that the M10 peptide may have potential for use in the treatment of scleroderma-associated interstitial lung disease and other forms of pulmonary fibrosis, for example, idiopathic pulmonary fibrosis," says Galina S. Bogatkevich, M.D., Ph.D.
When instilled intratracheally, bleomycin induces fibrotic changes in the lungs, including peribronchial and interstitial infiltration of inflammatory cells, thickening of alveolar walls, and the development of numerous fibrotic lesions with excess deposition of extracellular matrix protein. Using this bleomycin-induced model of lung fibrosis, the MUSC investigators evaluated the anti-fibrotic effects of M10, using a scrambled peptide as a control. The scrambled peptide had the same ten amino acids as M10 but arranged in a different order. Fibrosis was quantitated using the Ashcroft scale, which ranges from 0 (normal) to 8 (totally fibrotic).
As expected, mice receiving bleomycin plus a scrambled peptide showed greater than eight times more lung fibrosis than controls receiving saline and scrambled peptide (Ashcroft scores, 5.63±1.72 vs. 0.69± 0.35). However, Ashcroft scores dropped to 1.67±1.01 when mice were administered both bleomycin and M10, suggesting the anti-fibrotic potential of this peptide.
Because M10 was given on the same day as bleomycin, its anti-fibrotic effects are considered preventive. To establish the therapeutic anti-fibrotic efficacy of M10, Bogatkevich and her MUSC colleagues are planning experiments in which M10 will be administered a week after the instillation of bleomycin, when fibrotic damage has already occurred. If these additional experiments suggest therapeutic efficacy, Bogatkevich hopes to find an industry partner to help take M10 forward into clinical trials.
Many researchers speculate that there is a final common pathway to fibrosis in many different organ systems. If an anti-fibrotic agent is demonstrated to be effective in systemic sclerosis, which can affect many different organs, it could potentially hold promise for treating fibrotic disease that is confined to particular organ systems as well.
Bogatkevich and the other MUSC investigators also performed in vitro studies to assess the efficacy of M10 in decreasing abnormal collagen production/deposition and to shed light on the mechanism by which it does so. Skin and lung fibroblasts were obtained from three deceased patients with systemic sclerosis with confirmed lung involvement. As expected, these fibroblasts showed high levels of collagen production. Incubation of these fibroblasts with M10 reduced collagen expression in a dose-dependent manner. M10 likewise reduced collagen production induced in normal cells by administration of TGF-β1 without affecting baseline collagen levels.
These findings suggest that the anti-fibrotic effects of M10 may rely on its suppression of the TGF-β1 pathway. Indeed, protein interaction assays showed that M10 likely achieves such suppression by interacting with the Smad2 protein, thereby preventing it from binding to Smad3, which is necessary for the downstream inflammatory effects of the TGF-β1 pathway.
Original publication
Ilia Atanelishvili, Yuichiro Shirai, Tanjina Akter, Taylor Buckner, Atsushi Noguchi, Richard M. Silver, Galina S. Bogatkevich; "M10, a caspase cleavage product of the hepatocyte growth factor receptor, interacts with Smad2 and demonstrates antifibrotic properties in vitro and in vivo"; Translational Research; 2016