NOXXON’s SDF-1 inhibitor NOX-A12 completes Phase I
Advertisement
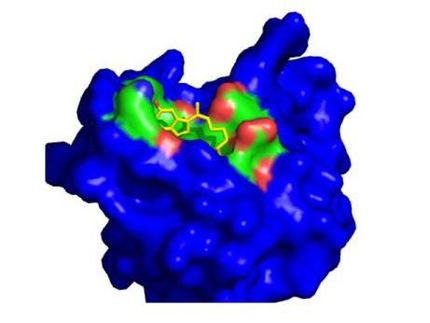
NOXXON Pharma announced the completion of Phase I single and multiple dose clinical trials of its SDF-1 inhibitor NOX-A12. In phase I studies with healthy volunteers single doses of NOX-A12 up to 10.8 mg/kg and daily doses up to 2 mg/kg for five days were found to be safe and well tolerated and resulted in dose-dependent mobilization of white blood cells and CD34+ cells as predicted by preclinical studies.
Based on compelling pre-clinical data in hematological and solid tumors, NOXXON believes that NOX-A12 has the potential to be developed for treatment of multiple oncology indications. Initiation of the first Phase IIa trial of NOX-A12 in a hematological tumor indication is planned early in 2012, subject to regulatory and ethics committee approvals.
Most read news
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.