Nanoaggregation on Command
Light-controlled reversible aggregation of microtubules mediated by paclitaxel-modified cyclodextrin
Advertisement
A combination of natural microtubules and synthetic macrocyclic receptors allows for the light-controlled, reversible aggregation of the microtubules into larger nanostructures. As Chinese scientists have reported in the journal Angewandte Chemie, when in a cellular environment these aggregated microtubules can also change cell morphology, causing cell death. The researchers hope to learn more about diseases caused by the improper aggregation of proteins.
© Wiley-VCH
In nature, the aggregation of molecules into superstructures plays an important role. Dynamic microtubules are protein filaments that combine with other components to form the cytoskeletons of our cells. During the cell cycle, microtubules constantly assemble and disassemble. Scientists from Nankai University and the Collaborative Innovation Center of Chemical Science and Engineering (Tianjin, China) conceived of the idea to combine microtubules into larger supramolecular aggregates using synthetic “receptors”. In this way they hoped to produce innovative biomaterials and gain new knowledge about biological aggregation processes.
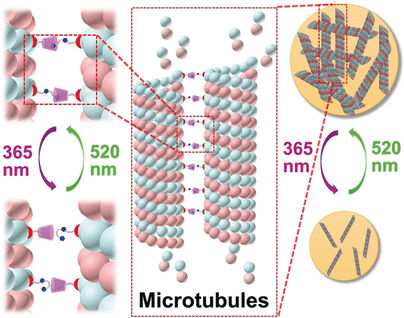
To attach the synthetic receptors to the microtubules, the team working with Yu Liu chose a cancer drug called paclitaxel. This molecule binds to microtubules and blocks deconstruction of the cytoskeleton, stopping cell division and causing cell death. As a receptor, the researchers chose a large cup-shaped molecule from the cyclodextrin family. These macrocyclic molecules can incorporate other molecules as “guests” in their large cavities. In this case the guest is an arylazopyrazole (AAP), a molecule with two aromatic rings bridged by a nitrogen-nitrogen double bond. The molecule can take one of two forms: a bent cis form and a straight trans form. Only the straight version fits into the cyclodextrin “cup”. The clever trick here is that, by using light of two different wavelengths, the AAP can be switched back and forth between its two forms at will.
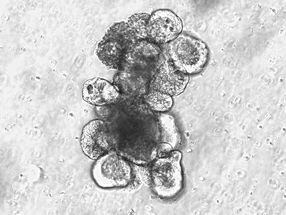
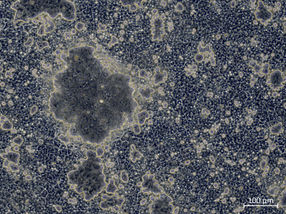
The researchers used paclitaxel as a connector to attach “cups” and their “guests” to microtubules. Irradiation with visible and UV light switches the microtubules between an aggregated and non-aggregated form, respectively, as demonstrated by spectroscopic and microscopic examination. The aggregates take on a broad range of morphological variations, ranging from nanofibers to nanoribbons and nanoparticles of different sizes.
It is particularly interesting that the aggregation of the microtubules can also be triggered within cells. This causes the cells to shrink and die, which demonstrates that the cytotoxic effect of paclitaxel can be increased significantly.
The researchers hope that their approach will increase our understanding of the processes involved in physiological and pathological protein nanoassembly, and could create new prospects in the treatment of diseases caused by the improper aggregation of proteins.