Peptides vs. superbugs
Advertisement
Several peptides have an antibacterial effect - but they are broken down in the human body too quickly to exert this effect. Empa researchers have now succeeded in encasing peptides in a protective coat, which could prolong their life in the human body. This is an important breakthrough because peptides are considered to be a possible solution in the fight against antibiotic-resistant bacteria.
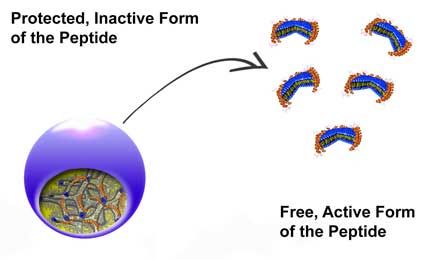
The peptides are located within the protective casing of the nanocarriers. The anti-microbial activity of the peptide is deployed when the structure of the nanocarrier is altered by external influences.
Empa
They occur in many organisms and constitute natural weapons against bacteria in the body, being known as antimicrobial peptides. They offer a possible – and now also urgently needed – alternative to conventional antibiotics, but have not yet been successfully used in a clinical context. The reason for this lies in their structure, which results in peptides being broken down relatively quickly inside the human body, before they can have an anti-bacterial impact.
In Empa's Biointerfaces Department in St. Gallen, a team led by Stefan Salentinig has now succeeded, in collaboration with the University of Copenhagen, in developing a kind of shuttle system made of liquid-crystalline nanomaterials (so-called nanocarriers), which protect the peptides and thus ensure they safely reach the target site. The specially developed nanocarriers consist of so-called structure-forming lipids, which can accommodate the antibacterial peptides and hold or release them based on the nature of the structure. Initial tests with bacterial cultures have shown that the peptides are completely enclosed by the nanocarriers and thus remain stable. However, once they are released they exert their full effect and prove extremely effective in fighting bacteria.
Peptides are good - peptides with nanocapsules are even better
The researchers have also documented an additional characteristic of the nanocarriers. Peptides are already effective against bacteria when working "alone" - but in combination with the carrier structure they are even stronger. Thus the protective casings formed by the lipids not only ensure the safe delivery of the peptides to the area where they are needed, but also intensify their impact at the target site. The research carried out by Empa and the University of Copenhagen could therefore be a first step in the successful fight against antibiotic-resistant bacteria, as peptides use a different mechanism from that used by antibiotics and destroy the membrane of bacteria. Even antibiotic-resistant superbugs are not equipped against such an effect. "Of course, the bacteria might eventually also adapt to be more resistant," commented Salentinig. However, this would not happen overnight and we would have a new weapon in the arsenal for the fight against multi-resistant bacteria.
In a next step, the researchers want to structure the nanocarriers in a way that enables them to take effect at a specific time. The peptides would therefore be protected within the nanostructure and then released when needed and as the result of an alteration in their structure. At the "press of a button", so to speak. This is especially important in the medical field, for example when treating open wounds or using catheters.