Anti-inflammatory principle also works against lung cancer and emphysema
Advertisement
Neither lung cancer nor emphysema, a chronic lung disease, were previously considered to be inflammatory diseases. Surprisingly, however, inflammatory processes play a role in both diseases, similar to, for example, those involved in the development of chronic inflammatory bowel disease or rheumatism. This has been revealed in studies by the working group led by Professor Brendan Jenkins at the Hudson Institute of Medical Research (Australia), in cooperation with the Kiel Biochemistry Professor Stefan Rose-John, from the Schleswig-Holstein-based Cluster of Excellence "Inflammation at Interfaces".
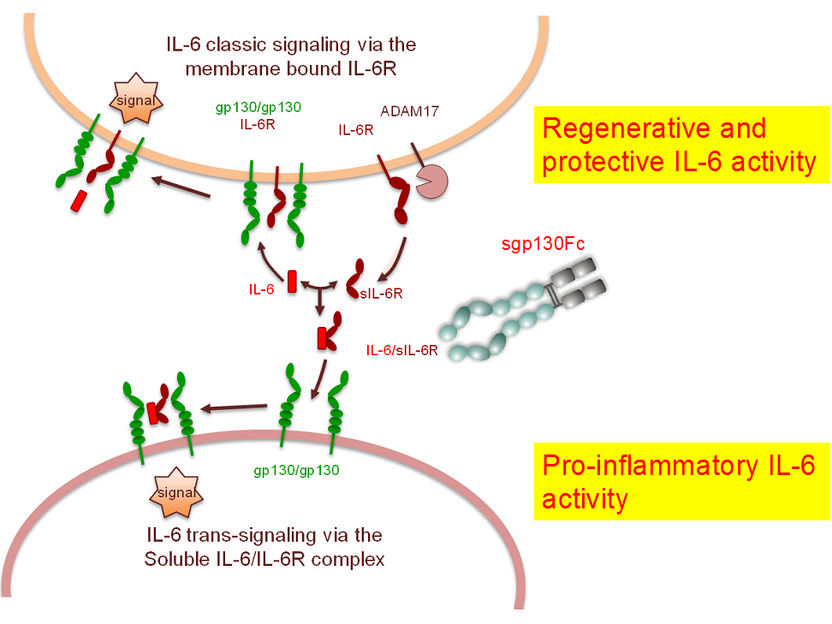
In principle, Interleukin-6 (IL-6) works via two different signal paths. In the “classical path”, IL-6 docks on to its receptor, which is bound to the cell membrane. This complex binds with gp130 and thereby triggers the signal to activate the immune system. This signal path is restricted to cells that carry an IL-6 receptor. This is only a few types of cells, however, primarily immune cells and liver cells. Nevertheless, other cells can also respond to the signal from IL-6. To enable this, IL-6 binds with the “soluble” IL-6 receptor found in the blood and in inflamed tissue. This second signal path, so-called trans-signalling, is decisive for inflammation processes in diseases. Externally-supplied sgp130Fc binds with the soluble complex, so that no trans-signalling is triggered.
Christian-Albrechts-Universität zu Kiel
Based on animal models, they were able to prove that the inflammatory messenger Interleukin-6 (IL-6) propels both lung diseases. Experiments showed that this takes place via a soluble IL-6 receptor with a special signal path, so-called IL-6 trans-signalling". At the same time, it was possible to use an IL-6 trans-signalling inhibitor to halt disease progression. These results, published in the American Journal of Respiratory and Critical Care Medicine (July 2016) and in Cancer Research (January 2016) enable new therapeutic options. Professor Stefan Rose-John is convinced of this: “This is really spectacular. I am sure that it is worthwhile to develop this further.”
In both studies, Professor Brendan Jenkins and his team showed that an inflammatory messenger, Interleukin 6 (IL-6), propels the disease progression through a signal pathway known as trans-signalling. The Kiel Biochemistry Professor Stefan Rose-John discovered this signal pathway around 20 years ago, and named it trans-signalling, in contrast to classical signalling. “The special thing about trans-signalling is that thereby Interleukin 6 also works on those cells that don't have their own IL-6 receptor,” explained Rose-John, who initiated the projects together with Brendan Jenkins. “What distinguishes my group, and what makes us highly valuable partners for many laboratories worldwide, is that we have the best tools available to do research in IL-6 signalling. Only with our tools you can answer the question of whether it takes place via the membrane-bound or soluble receptor, and this is important.”
The goal of this study – and further studies – is to identify additional diseases in which IL-6 is involved. In previous investigations into the inflammation of joints and intestine, as well as into the growth of cancer cells, it was determined that the disease-causing effects of Interleukin 6 are primarily conveyed via IL-6 trans-signalling. This has also been confirmed now by Jenkins’ studies on lung cancer and emphysema. “Trans-signalling and IL-6 have very important effects on lung diseases. They are the master regulators and can be specifically targeted by sgp130Fc,” explained the Australian immunologist, who was surprised by the close link between the two very different lung diseases. This is because emphysema is characterised by the loss of lung tissue, and lung cancer by uncontrolled growth of tissue.
The protein sgp130Fc is a potent inhibitor of IL-6 trans-signalling. It was developed at the Institute of Biochemistry at Kiel University under the leadership of Stefan Rose-John, and is currently being clinically tested as a therapy for people with chronic inflammatory bowel diseases. The studies, which have now been published show that, in principle, sgp130Fc is also a therapy option for people with certain lung cancer diseases (KRAS gene mutations) or with emphysema.
Anti-inflammatory effect – protein sgp130Fc
The special thing about sgp130Fc is: it specifically only switches off IL-6 trans-signalling (which triggers and propels chronic inflammation), without basically inhibiting all the other functions of IL-6. Additional important functions of Interleukin-6 in the immune system, the metabolism, and in liver regeneration, are largely unaffected. This makes it significantly different from other molecules which completely block Interleukin-6, such as the monoclonal antibody Tocilizumab, which is used in the therapy of Rheumatoid Arthritis.
The protein sgp130Fc imitates soluble gp130, which occurs in the blood naturally. However, by linking the gp130 molecules in pairs, it is significantly more effective than the natural soluble protein. Together with the soluble IL-6 receptor, it attaches to Interleukin-6 in the blood. The complex can therefore not bind with the membrane-bound gp130, and cannot trigger trans-signalling, thus inhibiting inflammation.
Original publication
Saleela M Ruwanpura, Louise McLeod, Lovisa F Dousha, Huei J Seow, Sultan Alhayyani, Michelle D Tate, Virginie Deswaerte, Gavin D Brooks, Steven Bozinovski, Martin MacDonald, Christoph Garbers, Paul T King, Philip G Bardin, Ross Vlahos, Stefan Rose-John, Gary P Anderson, and Brendan J Jenkins; "Therapeutic Targeting of the IL-6 Trans-signalling/mTORC1 Axis in Pulmonary Emphysema"; Am J Respir Crit Care Med; 2016
























































