Malaria-causing parasite manipulates liver cells to survive
Advertisement
When the malaria-causing Plasmodium parasite first slips into the human bloodstream, injected by the bite of an infected mosquito, it does not immediately target red blood cells.
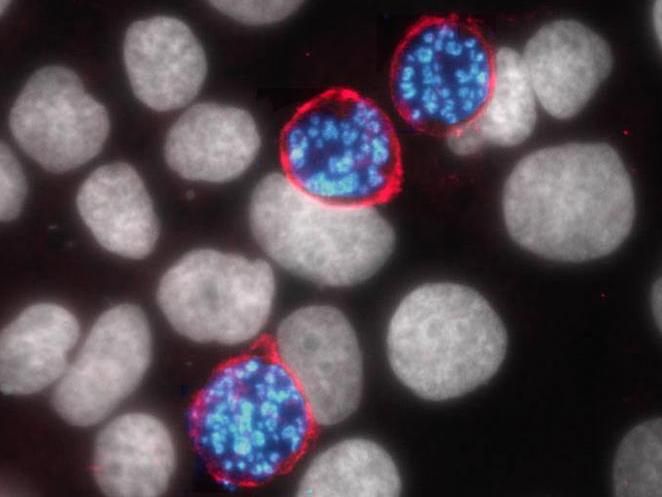
The liver stage of the malaria parasite Plasmodium (blue) relies on a host protein called aquaporin-3 (pink) to survive and copy itself. Inhibiting the function of aquaporin-3 may provide a new way to fight the proliferation of the Plasmodium parasite and prevent malaria before symptoms start.
Dora Posfai, Duke University
Instead, it seeks refuge inside the liver and rapidly reproduces, copying itself as many as 30,000 times in the span of 48 hours.
After building strength in numbers, the parasite leaves the liver and escapes into the blood stream, invading red blood cells and triggering the devastating disease.
The battle against malaria usually focuses on either helping people evade infected mosquitos or developing strategies to kill the parasite after it raids red blood cells. But a team of Duke University researchers wants to take a different tactic -- disrupting the parasite while it lurks inside the liver.
In a new study, the team shows that the Plasmodium parasite tricks liver cells into pumping out a protein called aquaporin-3, and then steals the protein for itself. Using an inhibitor to disable aquaporin-3 curtails the parasite's ability to reproduce inside the liver, the researchers report.
"This parasite found a way to manipulate the host's liver cells to make it favorable for this replication event," said Emily Derbyshire, an assistant professor of chemistry at Duke. "This suggests that maybe we can develop drugs to try to target the host to prevent malaria."
After arriving at the liver, Plasmodium forces its way into liver cells, stealing a bit of the cell membrane to form a small pouch inside the cell. This pouch, called a vacuole, provides a safe harbor while the parasite grows and divides, stealing nutrients and proteins from the host cell along the way.
"The liver stage is a checkpoint, a bottleneck, where it goes from a few dozen parasites to many thousands of parasites, which are released from the liver into the blood where it is amplified into hundreds of billions of parasites," said Peter Agre, Director of the Johns Hopkins Malaria Research Institute, who was not involved in the study.
"If we could put out the forest fire when it is the smallest possible little campfire, that would be a potential therapeutic breakthrough," said Agre, who won the 2003 Nobel Prize in Chemistry for the discovery of aquaporins.
But studying the liver stage in the lab is notoriously difficult. To infect liver cells, researchers must first isolate Plasmodium from the salivary glands of infected mosquitos where it only exists in small numbers.
Dora Posfai, a graduate student in the department of molecular genetics and microbiology at Duke, spent three-hour stretches dissecting infected mosquitos under a microscope, using a small needle to cut out their salivary glands and extract the parasites hidden inside.
After infecting human liver cells with the parasite, Posfai and Sandeep Dave, a professor of medicine in the Duke School of Medicine, used RNA sequencing to comb through all 20,000 genes in the human genome, searching for genes that are switched on in infected liver cells.
The team decided to investigate the role of one protein that they found in greater numbers called aquaporin-3 (AQP-3), a channel protein that sits astride cell membranes and plays a key role in shuttling water and nutrients into and out of the cell.
Liver cells do not normally produce AQP-3, instead relying on other types of aquaporin for water transport. But after infection with Plasmodium, the liver cells started producing the protein in droves.
The team used fluorescence imaging to track where all the new AQP-3 proteins were going -- and followed them straight to the vacuole membrane surrounding all the rapidly replicating Plasmodium cells.
"This is the first time we have seen upregulation of a human protein that is then trafficked to the vacuole membrane just to help the parasite," Derbyshire said.
When Posfai exposed the liver cells to an AQP-3 inhibitor called auphen, which blocks nutrients from passing through the port formed by aquaporin, she found the numbers of parasites decreased substantially.
"This is a great proof-of-principle that you can develop small molecules to fight Plasmodium in the liver, and one could now have a campaign looking specifically for inhibitors against this protein," Derbyshire said.
Barring the Plasmodium parasite from reproducing in the liver could not only help treat malaria before the onset of symptoms, but also side-step the development of drug-resistant strains by targeting proteins in the host cells rather than in the rapidly evolving parasite.
"We do have medicines for treating malaria in the blood, but we don't have good medicines for treating it in the liver," Agre said. "Knowing a new, important target like AQP-3 could lead to the discovery of new medicines."
Original publication
Dora Posfai, Kayla Sylvester, Anupama Reddy, Jack G. Ganley, Johannes Wirth, Quinlan E. Cullen, Tushar Dave, Nobutaka Kato, Sandeep S. Dave, Emily R. Derbyshire; "Plasmodium parasite exploits host aquaporin-3 during liver stage malaria infection"; PLOS Pathogens; 2018