Method of the future uses single-cell imaging to identify gene interactions
Advertisement
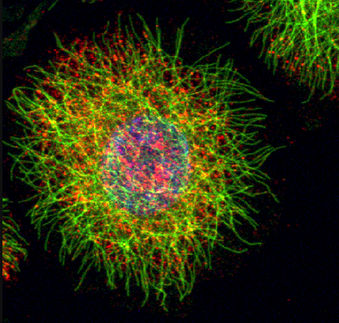
cellular imaging offers a wealth of data about how cells respond to stimuli, but harnessing this technique to study biological systems is a daunting challenge. In a study published in Genome Research, researchers have developed a novel method of interpreting data from single-cell images to identify genetic interactions within biological networks, offering a glimpse into the future of high-throughput cell imaging analysis.
For years, scientists have been peering through a microscope at cells as they change appearance in response to different treatments, yet data collection is arduous, largely conducted qualitatively by eye. However, recent technological advances have led to the development of high-throughput image screening methods that can produce extensive datasets of hundreds of different morphological features.
With the ability to collect large imaging datasets, researchers from MIT and Harvard Medical School recognized an opportunity to explore the cellular networks that regulate cell morphology. “These images are an enormous source of data that is only beginning to be tapped,” said MIT researcher Bonnie Berger, senior author of the work published today. “We realized we had enough data to go beyond classification and start to understand the mechanism behind the differences in shape.”
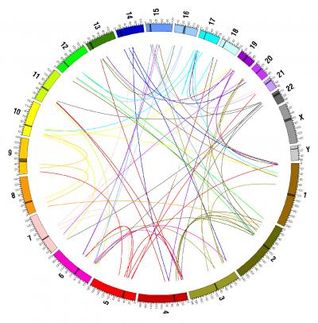
To meet the challenge of interpreting cell image data, Berger and MIT graduate student Oaz Nir developed a novel computational model to identify genetic interactions using high-dimensional morphological data. Integrating prerequisite knowledge of a pathway, their model maps potential interactions within a network by looking for similar morphological features upon genetic perturbation.
The group demonstrated the method by analyzing the Rho-signaling network in fruit flies, a network that regulates cell adhesion and motility in eukaryotic organisms. In collaboration with Chris Bakal and Norbert Perrimon at Harvard Medical School, they “knocked-down” components of the Rho-signaling network using RNA interference and then imaged thousands of fly cells, gathering measurements of cell perimeter, nuclear area, and more than 150 other morphological features for each cell. The data was then passed through the computational framework to produce a set of high-confidence interactions, supported by confirmation of previously known interactions.
The group found that by making combinatorial knockdowns of Rho network components, their computational method was able to accurately infer Rho-signaling network interactions more precisely than when using only data from single knockdowns. Berger noted that this finding highlights the importance of combinatorial experiments for inferring complex networks, necessary to overcome natural redundancy in signaling pathways. As perturbation of the Rho pathway in humans has been implicated in cancer and other diseases, the authors believe that these predicted interactions will be excellent candidates for future study.
Berger expects that in combination with other sources of data, imaging as a new source of high-throughput data should appreciably increase the accuracy of known signaling networks. “This work provides a glimpse into the future,” added Berger, “where looking under the microscope manually at cells one-by-one is replaced with automated high-throughput processing of many cellular images.”
Original publication: Nir O, Bakal C, Perrimon N, Berger B.; "Inference of RhoGAP/GTPase regulation using single-cell morphological data from a combinatorial RNAi screen."; Genome Res 2010.