Milestone: Researchers have generated primordial germ cells from stem cells – a world’s first
A big step toward producing rhino gametes
Advertisement
To save the northern white rhinoceros from extinction, the BioRescue team is racing to create lab-grown egg and sperm cells of the critically endangered subspecies. The team has now reported a milestone in “Science Advances”: they have generated primordial germ cells from stem cells – a world’s first.

The last two females live in the Kenyan wildlife reserve "Ol Pejeta".
Jan Stejkal, Safari Park Dvůr Králové
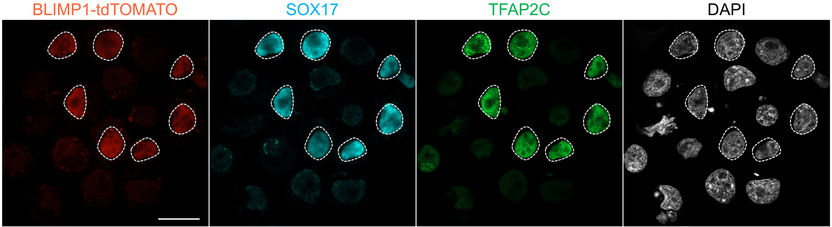
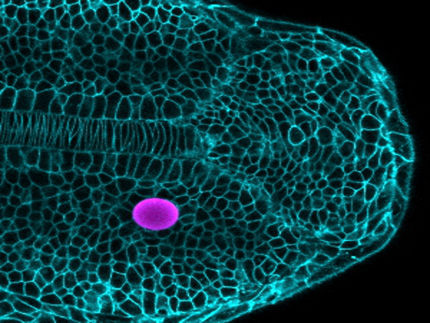
The SOX17 gene played a key role in inducing primordial germ cells from pluripotent stem cells of the white rhinoceros.
Masafumi Hayashi, Universität Osaka

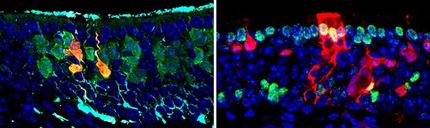
Thirty-three-year-old Najin and her daughter Fatu are the last surviving northern white rhinos on the planet. They live together in a wildlife conservancy in Kenya. With just two females left, this white rhino subspecies is no longer capable of reproduction – at least not on its own. But all hope is not lost: according to a paper published in the journal “Science Advances”, an international team of researchers has successfully cultivated primordial germ cells (PGCs) – the precursors of rhino eggs and sperm – from embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs).
This represents a major milestone in an ambitious plan. The BioRescue project, which is coordinated by the Leibniz Institute for Zoo and Wildlife Research (Leibniz-IZW) and has been funded by the German Federal Ministry of Education and Research (BMBF) since 2019, wants to save the northern white rhino from extinction. To this end, the scientists are pursuing two strategies – one of them trying to generate viable sperm and eggs from the skin cells of deceased rhinos. The idea is to implant the resulting embryos into closely related southern white rhino females, who will then carry the surrogate offspring to term. And so the northern white rhino subspecies, which humans have already effectively wiped out through poaching, may yet be saved thanks to state-of-the-art stem cell and reproductive technologies.
First success with an endangered species
To get from a piece of skin to a living rhinoceros may be a true feat of cellular engineering, but the process itself is not unprecedented: the study’s co-last author Professor Katsuhiko Hayashi leads research labs at the Japanese universities of Osaka and Kyushu in Fukuoka, where his teams have already accomplished this feat using mice. But for each new species, the individual steps are uncharted territory. In the case of the northern white rhinoceros, Hayashi is working in close cooperation with Dr. Sebastian Diecke’s Pluripotent Stem Cells Technology Platform at the Max Delbrück Center and with reproduction expert Professor Thomas Hildebrandt from Leibniz-IZW. The two Berlin-based scientists are also co-last authors of the current study.
“This is the first time that primordial germ cells of a large, endangered mammalian species have been successfully generated from stem cells,” explains the study’s first author, Masafumi Hayashi of Osaka University. Previously, it has only been achieved in rodents and primates. Unlike in rodents, the researchers have identified the SOX17 gene as a key player in rhinoceros PGC induction. SOX17 also plays an essential role in the development of human germ cells – and thus possibly in those of many mammalian species.
The southern white rhino embryonic stem cells being used in Japan come from the Avantea laboratory in Cremona, Italy, where they were grown by Professor Cesare Galli’s team. The newly derived northern white rhino PGCs, meanwhile, originated from the skin cells of Fatu’s aunt, Nabire, who died in 2015 at Safari Park Dvůr Králové in the Czech Republic. Diecke’s team at the Max Delbrück Center was responsible for converting them into induced pluripotent stem cells.
Next step: cell maturation
Masafumi Hayashi says that they are hoping to use the cutting-edge stem cell technology from Katsuhiko Hayashi’s lab to save other endangered rhino species: “There are five species of rhino, and almost all of them are classified as threatened on the IUCN Red List.” The international team also used stem cells to grow PGCs of the southern white rhino, which has a global population of around 20,000 individuals. In addition, the researchers were able to identify two specific markers, CD9 and ITGA6, that were expressed on the surface of the progenitor cells of both white rhino subspecies. “Going forward, these markers will help us detect and isolate PGCs that have already emerged in a group of pluripotent stem cells,” Hayashi explains.
The BioRescue scientists must now move on to the next difficult task: maturing the PGCs in the laboratory to turn them into functional egg and sperm cells. “The primordial cells are relatively small compared to matured germ cells and, most importantly, still have a double set of chromosomes,” explains Dr. Vera Zywitza from Diecke’s research group, who was also involved in the study. “We therefore have to find suitable conditions under which the cells will grow and divide their chromosome set in half.”
Genetic variation is key for conservation
Leibniz-IZW researcher Hildebrandt is also pursuing a complementary strategy. He wants to obtain egg cells from 22-year-old Fatu and fertilize them in Galli’s lab in Italy using frozen sperm collected from four now deceased northern white rhino bulls. This sperm is thawed and injected into the egg in a process known as intracytoplasmic sperm injection (ICSI). However, Hildebrandt explains that Fatu is not able to bear her own offspring, as she has problems with her Achilles tendons and cannot carry any additional weight. Her mother Najin, meanwhile, is past child-bearing age and also suffers from ovarian tumors. “And in any case, since we only have one donor of natural eggs left, the genetic variation of any resulting offspring would be too small to create a viable population,” he adds.
The team’s top priority, therefore, is turning the PGCs they now have at their disposal into egg cells. “In mice, we found that the presence of ovarian tissue was important in this crucial step,” Zywitza explains. “Since we cannot simply extract this tissue from the two female rhinos, we will probably have to grow this from stem cells as well.” The scientist is hopeful, however, that ovarian tissue from horses could come in useful, as horses are among the rhinos’ closest living relatives from an evolutionary standpoint. If only humans had taken as good care of the wild rhino as they had of the domesticated horse, the immense challenge now facing the BioRescue scientists could perhaps have been avoided altogether.