IBM and Cellomics Create Affordable Technology Solution to Accelerate Cell-Based Drug Discovery
First High-Content Screening and Analysis Solution Being Used by Bio-Pharmaceutical Researchers at EPOPLUS to Identify Off-Target Effects in Live Cells
Advertisement
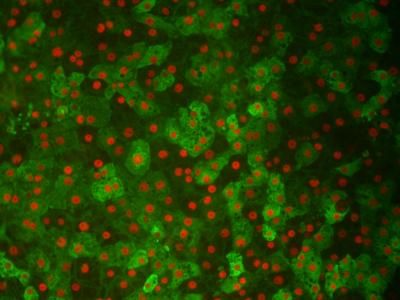
Somers and Pittsburgh. IBM and Cellomics, Inc., a leader in creating software and hardware tools for High Content Screening (HCS), today announced they have launched a customized information technology (IT) solution that allows biopharmaceutical scientists and academic researchers to better understand how potential drug candidates or targets of interest affect cellular function.
The new solution, which allows for high-content screening and in-silico modeling is already being installed at EPOPLUS, GMBH & Co. KG. -- a biopharmaceutical company developing a novel Low-Dose-Erythropoietin therapy likely to have a major affect on the understanding of degenerative diseases, stem cell behavior and cell biology.
"We are very pleased to form this collaboration with IBM and Cellomics -- the world's leading companies in Information Technologies and High Content Screening ," said Dr. Ferdinand Bahlmann, president of EPOPLUS. "The expertise and technology they provide greatly reduce the significant cost associated with screening and evaluating potential cell therapeutics."
By partnering with Cellomics, IBM is furthering its work in the field of information-based medicine, the process of improving existing pharmaceutical and medical practices with knowledge about the human genome. The rich contextual data that can be obtained about cellular functions using HCS generates information necessary for modeling and simulation that empowers biopharmaceutical organizations to design more accurate trials, reducing time and cost spent making new drugs.
"In working with Cellomics to build this joint, turnkey solution, we're able to help organizations like EPOPLUS enhance their cellular research capabilities," said Mike Svinte, vice president, information-based medicine, IBM. "By putting this technology together in an out-of-the-box solution, we are hoping to give many more researchers the ability to test potential drug compounds against crucial, contextual information about cell structure, function and interaction."
With the goal of delivering an IT solution that can automate and manage large volumes of
data, IBM and Cellomics have combined Cellomics patented High Content Informatics (HCi(TM))
software with proven servers, storage and management software from IBM. The resulting
HCi Appliance offers a scalable architecture that includes the Tivoli(R) suite of security
compliance products, IBM
- Cellomics(R) Store: an enterprise-level database that stores and manages images and image-related data
- Cellomics HCS Applications Server: provides a middleware layer that enables communication between the database and analysis tools, and establishes a framework for plug-in modules and additional functionality
- vHCS(TM) Discovery Toolbox: offers users a full suite of tools to manage, analyze and visualize HCS data from any licensed HCS instrument, including the KineticScan(R) HCS Reader and ArrayScan(R) VTI HCS Reader
"High-content screening generates hundreds of data points per cell. This vast amount of data needs to be properly managed, stored, archived and protected if researchers are to benefit from cellular information," says R. Terry Dunlay, vice president of informatics at Cellomics. "Cellomics' innovative technology allows researchers to analyze and interpret data, and IBM supports this technology and ensures it operates at peak performance by providing industry expertise and robust, security-rich servers and data management storage solutions."