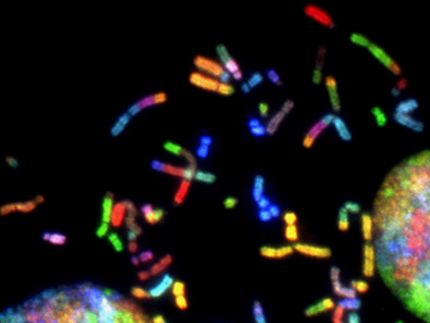
Captain T Cell Closes Financing Round to Advance its Proprietary, Next-Generation TCR-T Pipeline Into the Clinic
Developing novel solid tumor treatments addressing a high unmet medical need
Advertisement
Captain T Cell, a biotechnology company developing next-generation TCR-T cell therapies for solid tumors, announced the successful closing of an equity financing round with both new and existing investors. The latest equity funding, along with already secured grants, provides Captain T Cell with a total budget of EUR 20 million to advance its best-in-class autologous program CTC127 to clinical Phase I read-outs and its first-in-class allogeneic program for off-the-shelf solid tumor treatments towards clinical trial readiness.
The round was led by Springboard Health Angels and Pluton Asset Holding AG, joined by Sintra Limited and Technologiegründerfonds Sachsen, as well as existing investors i&i Biotech Fund, HIL-INVENT and Brandenburg Kapital.
The funds will enable the Company´s lead program CTC127 to progress into a first-in-human Phase I clinical trial (TOMATA - “Toolbox-modified MAGE-A4-TCR-T cell therapy for HLA-A1”). The trial will be led by principal investigator Prof. Antonia Busse, Charité – Universitaetsmedizin Berlin, with participation from eight other leading cancer centers across Germany. The study will enroll patients with multiple advanced MAGE-A4-positive solid tumor indications, including lung, bladder, gastroesophageal, ovarian, and head and neck cancers. CTC127 is based on the Company’s proprietary and versatile engineering toolbox, which enables functional “armoring” of T cells to enhance their anti-tumor activity, persistence, and resilience within the solid tumor microenvironment.
In parallel, the Company has already demonstrated complete solid tumor responses in vivo with a program leveraging its first-in-class allogeneic TCR-T cell platform and will advance it toward clinical trial readiness. Furthermore, the Company plans to extend its proprietary best-in-class toolbox towards in vivo T cell therapy applications.
“Despite a challenging fundraising environment, we have gained broad investor support, which underscores the strong competitive profile of Captain T Cell’s best- and first-in-class next-generation T cell therapy platform and enables us to advance these therapies for the benefit of cancer patients,” said Dr. Felix Lorenz, CEO of Captain T Cell. “Our best-in-class autologous program CTC127 will enter the fully funded TOMATA clinical trial and our first-in-class allogeneic TCR-T platform is progressing toward clinical readiness. We are optimistic that our best-in-class toolbox technologies will be clinically validated in the upcoming trial and subsequently expanded into off-the-shelf approaches.”
“We are excited to co-lead this recent financing round of Captain T Cell,” said Dr. Andreas Schmidt of Springboard Health Angels. “The Company´s lead program CTC127 holds the potential to become a highly effective and safe therapy for solid tumor patients who urgently need novel treatment options. With funding secured through the upcoming Phase I readout, Captain T Cell is well positioned for a major value inflection.”
Dr. Theis Terwey, representing lead investor Pluton Asset Holding AG, commented: “MAGE-A4 is a de-risked and commercially validated target. The available preclinical data convincingly demonstrate that Captain T Cell’s toolbox-enhanced approach is conceptually superior to on-market cell therapies against solid tumors, providing strong evidence that the Company can deliver competitive efficacy in large solid tumor indications.”
Dr. Barbora Šumová, Investment Director at i&i Biotech Fund, added: “Since our initial investment, the Captain T Cell team has consistently executed with exceptional speed and quality, achieving every milestone set at the seed round funding stage. We are proud to continue supporting the Company as it advances towards becoming a clinical-stage company.”
Prof. Antonia Busse, Principal Investigator at Charité – Universitaetsmedizin Berlin, noted: “There remains a profound unmet need for effective therapies for patients with advanced solid tumors. We look forward to initiating the clinical safety and efficacy evaluation of CTC127 together with eight other leading cancer centers in Germany.”
Other news from the department business & finance
Most read news
More news from our other portals
Something is happening in the life science industry ...
This is what true pioneering spirit looks like: Plenty of innovative start-ups are bringing fresh ideas, lifeblood and entrepreneurial spirit to change tomorrow's world for the better. Immerse yourself in the world of these young companies and take the opportunity to get in touch with the founders.