The fork in the road to DNA repair
Advertisement
The human body consists of trillions of cells, and within each are billions of DNA molecules. Strict maintenance of the molecules is essential to maintain a healthy cell and thus a healthy body.
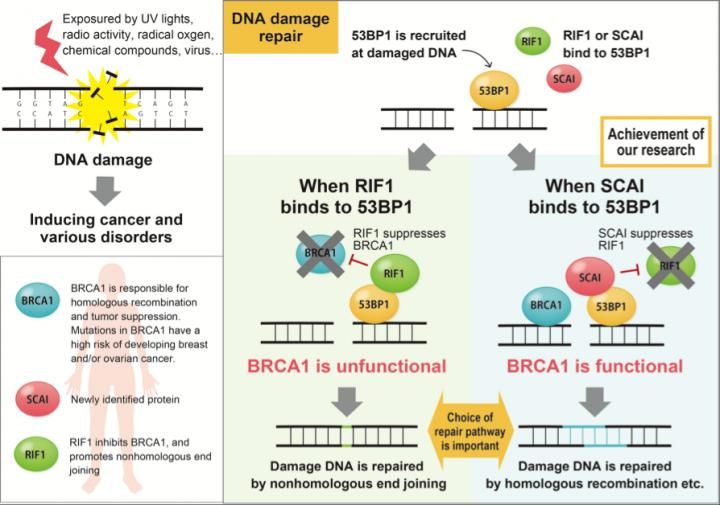
DNA damage repair is shown.
Osaka University
This maintenance is challenged by the daily bombardment of chemicals, UV light, radical oxgen and radiation that can damage the DNA molecules. If left unrepaired, the damage could lead to genomic instability and cell death. Thankfully, evolution has created in the cell innate repair mechanisms to fix any damaged DNA.
"The two mechanisms in the cell are non-homologous end joining (NHEJ) and homologous recombination (HR) for repairing DNA double strand break" explains Chikashi Obuse, Professor at the Osaka University Graduate School of Sciences.
While NHEJ and HR both function to repair damaged DNA, they respond to different situations; types of damage, presence or absence of homologous template or cell cycle stages etc. What has continued to elude researchers is how the cell knows which system to call. Obuse shows in his latest report that the protein suppressor of cancer cell invasion (SCAI) plays an important role for the selection of HR.
To study the function of SCAI, Obuse and his team of scientists exposed human cells to X-ray irradiation to damage the DNA.
"Our results suggested SCAI bound to 53BP1 to promote the recruitment of HR proteins. When we depleted SCAI these proteins did not accumulate," he said.
In particular, Obuse highlighted the great diminishment of the protein BRCA1 at damage sites when SCAI was depleted. On the other hand, SCAI presence inhibited another protein, RIF1, to promote the recruitment of BRCA1.
"RIF1 is known to inhibit BRCA1 accumulation at DNA damaged sites. It binds to 53BP1. When we looked at confocal imaging of cells, we saw RIF1 initially accumulated at sites of DNA damage but was gradually replaced by SCAI," said Obuse.
This led the scientists to wonder if SCAI and RIF1 competed to bind to 53BP1 and whether this binding determined the DNA repair mechanism.
Indeed, additional experiments showed that the phosphorylation state of 53BP1 determined its binding partner.
"The next question for us is to determine which upstream kinases are responsible for phosphorylating the sites of 53BP1 needed for binding with SCAI," added Obuse. "The upstream signaling molecules will be important for helping to determine the cell's choice for either the NHEJ or HR pathway."