How Does Alzheimer's Disease Begin?
How the calcium balance gets out of control
Advertisement
Alzheimer's disease is one of the most complex and protracted diseases of the human brain. Especially the onset of the disease proves to be extremely difficult to study. This is 15 to 30 years before patients become clinically conspicuous due to cognitive deficits, especially in the areas of language and memory. It is known that a disturbed calcium balance in neurons is present at the beginning of Alzheimer's disease and probably contributes to the early memory failures. Professor Martin Korte and his team at the Technische Universität Braunschweig have shown in a study how the calcium balance gets out of control. The results have just been published in the journal "PNAS".
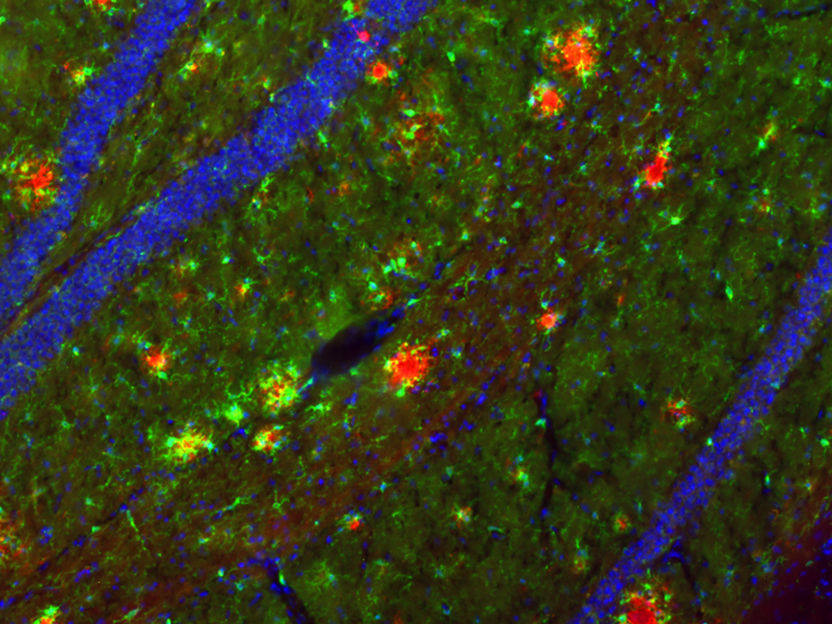
Aβ deposits have been visualized in red in the mouse hippocampus. In order to be able to examine these Alzheimer-typical deposits in the mouse, special mouse models are necessary.
Holz, Korte/TU Braunschweig
Characteristic of Alzheimer's disease are plaques - protein deposits outside nerve cells in the brain. These plaques are formed by a clumping of protein fragments consisting of the famous amyloid ß (Aß). But how is this molecule formed? It is cut out of a precursor protein in a complex two-step process. But that's not all: from this precursor protein – let's politely call it by its full name: Amyloid-Precursor Protein (APP) – there are also two sibling molecules (also known as homologues), for which only numbers remained to give them their names: APLP1 and APLP2.
In a recent study, Professor Dr. Martin Korte, together with Dr. Susann Ludewig and other members of his team from the Technical University of Braunschweig, was able to identify a mechanism how the amyloid precursor protein (APP), its cleaving products and its homologue (APLP2) control neuronal calcium currents. And it is not Aß that plays a role here, but another naturally occurring cleavage product of APP: the secreted APPsalpha (APPsα) is, according to the findings of the study, an essential regulator of the cellular calcium balance.
Calcium: Important for signal transmission in the brain
Calcium plays a fundamental role for the equilibrium-maintaining (homeostatic) as well as for the functional changes of neurons through altered neuronal activity (called plasticity), as it mediates and controls enzymatic signalling cascades up to gene transcription. The hypothesis, which neuroscientists at the Technical University of Braunschweig believe is strengthened by the new findings, is that the calcium balance in Alzheimer's disease, as well as the calcium dynamics, is already disturbed at the early onset of the disease.
Imbalance to the disadvantage of the calcium regulator
This imbalance is caused by an increased cleavage of APP - possibly due to ageing or chronic inflammatory processes - in the so-called "amyloidogenic signalling pathway" to Aß, i.e. to the protein fragments that are responsible for the famous extracellular Alzheimer associated plaques. This mechanism is at the expense of the calcium regulator APPsα, because the cleavage pathways are mutually exclusive. The Aß peptides can assemble into fibrous structures called fibrils and form molecular clumps.
The consequences of altered calcium permeability
This deposition between neurons leads to an altered calcium permeability of voltage-dependent calcium channels. In addition, synaptic molecules that serve as docking sites for the neurotransmitter glutamate and are associated with the capacity of higher brain functions such as learning and memory are altered. These are NMDA receptors (associated with calcium permeable ion channels in the cell membrane), and are removed from the synaptic membrane as a result of Aß deposition. This then sets in motion further cascades of negative effects that permanently disrupt signal transmission between neurons.
Starting point of the study
While numerous studies to date have examined the consequences of calcium currents impaired by Aß, there are very few studies devoted to the physiologically important role of APP, its homologues and functional domains. Korte and co. have addressed this in their current study, published in the internationally renowned journal of the American Academy of Sciences, PNAS. The results suggest that not only the increased release of Aβ can be regarded as a risk for the development of Alzheimer's dementia: At the onset of the disease, it is more likely that the reduced production of APPsα is the cause of the impaired calcium balance and its dynamic.
Calcium dynamics and calcium storage function are restricted
Furthermore, the team of authors, which includes the renowned Alzheimer's researcher Professor Ulrike Müller, who teaches at the University of Heidelberg, was able to show that together with the APP protein, the related APLP2 controls the calcium dynamics as well as the refilling of the internal calcium stores (called endoplasmic reticulum, ER) within the neurons in a previously unknown way.
Disruption of the adaptive abilities of brain cells
In addition, all this leads to a disruption of synaptic plasticity, i.e. the ability of synapses and neurons to optimize their circuitry properties during learning events. These cellular processes of synaptic change, which are so important for learning and memory processes, are disturbed above all by the fact that a transport molecule, which pumps calcium in large quantities into internal stores, no longer functions properly - and is also produced in altered quantities (in technical jargon: the function and expression are disturbed). This pump, named serca-ATPase, is responsible for pumping released calcium within the cell back into the internal storage in order to then be able to release calcium quickly and in large quantities within the neuron during stimulus transmission. Furthermore, the neuroscientists found that the expression of two calcium channel-associated proteins (Stim1 and Stim2) is altered in the absence of APP and APLP2.
Attempt to restore balance in APP cleavage through stimulated formation of APPsα
In this study, long-term expression of APPsα was used to try to restore the balance in APP cleavage. This should prevent or alleviate the symptoms of the disease - at least in the mouse model of Alzheimer's disease. It was observed that only a long-term, compensatory expression of APPsα was able to restore the physiologically important calcium balance. Furthermore, the previously impaired synaptic plasticity could be restored. APPsα normalized and rescued the normal expression of Serca ATPase and the proteins Stim1 and 2, which subsequently also restored the calcium concentration within the internal calcium stores (ER) to a healthy level.
The effectiveness of APPsα via gene therapy had already been demonstrated in animal models of Alzheimer's disease (published in Fol et al. Acta Neuropathol 2016, again with the participation of TU researchers). Here, the peptide was administered either acutely or chronically and was able to eliminate the dementia-typical impairments in synaptic plasticity and in the following memory processes.
Conclusion
In summary, this study demonstrates a central and important role of the APP protein family, specifically the extracellularly released fragment APPsα of the APP protein, in calcium homeostasis. These findings complement the already known growth-promoting and neuroprotective function of APPsα in neurons and add o this knowledge the new findings that APPsα is also involved in the regulation of calcium dynamics. It thus highlights the therapeutic potential of this domain for Alzheimer's disease therapy.