How a protein promotes cancer progression
Advertisement
Researchers at Sanford Burnham Prebys Medical Discovery (SBP) and the Technion in Israel have found a new role for the SHARPIN protein. In addition to being one of three proteins in the linear ubiquitin chain assembly complex (LUBAC), regulating NFκB and other inflammatory molecules, SHARPIN modulates PRMT5, an epigenetic master switch that controls several proteins linked to melanoma.
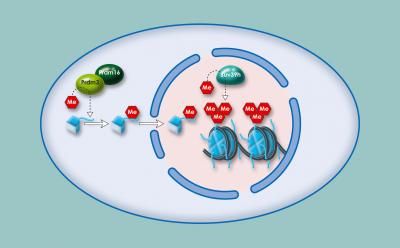
SHARPIN counteracts PRMT5 repression, its overexpression coincides with poor survival in MTAP-deleted melanomas.
Ronai Lab, Sanford Burnham Prebys Medical Discovery Institute (SBP)
"More studies are showing how important PRMT5 is in cancer, and our research highlights another layer of regulation of this very important enzyme," says Ze'ev Ronai, Ph.D., professor in the Tumor Initiation and Maintenance Program at SBP's NCI-designated Cancer Center and senior author on the paper. "This shows the complexity of PRMT5 regulation and points to a new direction in targeting PRMT5."
SHARPIN, is best known for its role in LUBAC, which plays a direct role in inflammatory signaling. However, SHARPIN has a second life, regulating PRMT5, which methylates individual genes and the histones that package DNA, controlling a wide range of gene expression. PRMT5 has been linked to prostate, breast, lung and other cancers.
Using human melanoma cell lines, the researchers found that SHARPIN (but not fellow LUBAC proteins HOIP and HOIL-1L) increases PRMT5 activity, which boosts transcription factors SOX10, PAX3 and MITF, all of which contribute to melanoma growth.
The researchers believe SHARPIN fine-tunes PRMT5, affecting how the enzyme methylates certain proteins, but not histones.
"SHARPIN actually dictates PRMT5 activity in a way we didn't expect," says Ronai. "It serves as a navigator, showing PRMT5 where to go and thus which proteins to methylate."
SHARPIN becomes especially important in tumors that have lost proteins CDKN2A and MTAP, which are co-deleted in around 15 percent of all cancers. When MTAP is deleted, PRMT5 activity is reduced. However, in those cases SHARPIN acts as a counterbalance, boosting PRMT5. Epidemiological data show that patients whose tumors lack MTAP, and have elevated levels of SHARPIN, have poorer prognosis.
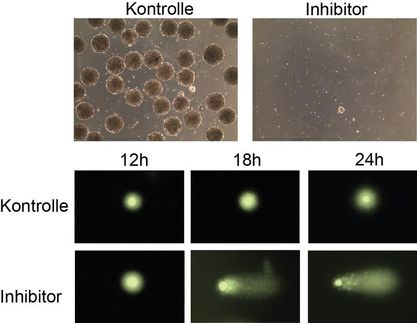
"Inhibiting SHARPIN in these tumor cells makes them much more susceptible to death," says Hyungsoo Kim,P.D., who co-led the study.
Pharmaceutical companies have been racing to develop anti-PRMT5 drugs, but these findings underscore the complexity of the pathway. It's quite possible that PRMT5 inhibitors and SHARPIN could end up in a tug of war, and SHARPIN inhibitors may be necessary to boost overall effectiveness.
"We may need to consider a possible combination of therapies," adds Ronai. "PRMT5 inhibition by itself may not be sufficient, and we may thus want to consider combination therapies to achieve more complete inhibition."
Original publication
Hironari Tamiya, Hyungsoo Kim, Oleksiy Klymenko, Heejung Kim, Yongmei Feng, Tongwu Zhang, Ji Yun Han, Ayako Murao, Scott J. Snipas, Lucia Jilaveanu, Kevin Brown, Harriet Kluger, Hao Zhang, Kazuhiro Iwai, Ze’ev A. Ronai; "SHARPIN-mediated regulation of protein arginine methyltransferase 5 controls melanoma growth"; JCI; 2017