Face of the future
Advertisement
A new technique developed by Gordana Vunjak-Novakovic, the Mikati Foundation Professor of biomedical engineering at Columbia Engineering and professor of medical sciences (in Medicine) at Columbia University, repairs large bone defects in the head and face by using lab-grown living bone, tailored to the patient and the defect being treated. This is the first time researchers have grown living bone that precisely replicates the original anatomical structure, using autologous stem cells derived from a small sample of the recipient's fat.
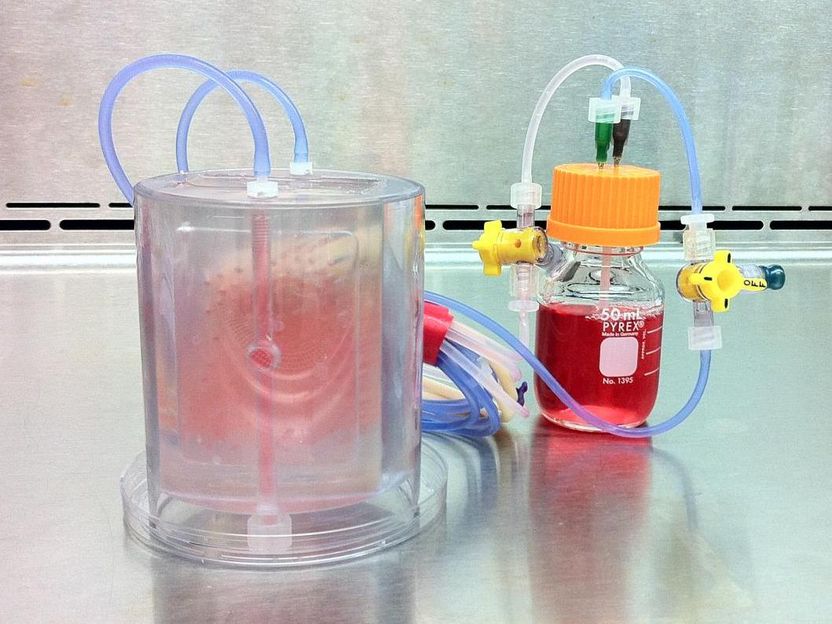
This is a perfused bioreactor with cultured bone seen inside.
Sarindr Bhumiratana/Columbia Engineering
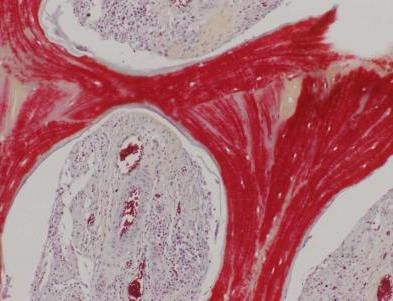
This image shows regenerated bone, with mineralized matrix (red) and strong vascular supply (vessels with red blood cells seen in cavities).
Sarindr Bhumiratana/Columbia Engineering

"We've been able to show, in a clinical-size porcine model of jaw repair, that this bone, grown in vitro and then implanted, can seamlessly regenerate a large defect while providing mechanical function," says Vunjak-Novakovic, who is also the director of Columbia's Laboratory for Stem Cells and Tissue Engineering, co-director of the Craniofacial Regeneration Center, and director of the Bioreactor Core of the NIH Tissue Engineering Center. "The need is huge, especially for congenital defects, trauma, and bone repair after cancer surgery. The quality of the regenerated tissue, including vascularization with blood perfusion, exceeds what has been achieved using other approaches. So this is a very exciting step forward in improving regenerative medicine options for patients with craniofacial defects, and we hope to start clinical trials within a few years."
Vunjak-Novakovic's team, which included researchers from Columbia Engineering's Department of Biomedical Engineering, Columbia's College of Dental Medicine, Louisiana State University, and Tulane University School of Medicine, fabricated a scaffold and bioreactor chamber based on images of the weight-bearing jaw defect, to provide a perfect anatomical fit. The scaffold they built enabled bone formation without the use of growth factors, and also provided mechanical function, both of which are unique advantages for clinical application. They then isolated the recipient's own stem cells from a small fat aspirate and, in just three weeks, formed the bone within a scaffold made from bone matrix, in a custom-designed perfused bioreactor. To mimic the logistics of envisioned clinical applications, where the patient and the bone manufacturing are at remote locations far from each other, the researchers shipped the bioreactor with the living bone across the country to be implanted.
An unexpected outcome was that the lab-grown bone, when implanted, was gradually replaced by new bone formed by the body, a result not seen with the implantation of a scaffold alone, without cells. "Our lab-grown living bone serves as an 'instructive' template for active bone remodeling rather than as a definitive implant," says Vunjak-Novakovic. "This feature is what makes our implant an integral part of the patient's own bone, allowing it to actively adapt to changes in the body throughout its life."
Vunjak-Novakovic and her team are now including a cartilage layer in the bioengineered living bone tissue to study bone regeneration in complex defects of the head and face. They are also advancing their technology through advanced preclinical trials, and in planning stages with the FDA for clinical trials, through her company epiBone.
"Having a chance to work on innovative research that may be part of our future is intriguing, energizing, and really inspiring," says the study's lead author Sarindr Bhumiratana PhD'12, who also is chief scientific officer at epiBone.
"Today, tissue engineering is truly changing the way we approach tissue repair, drug testing, disease modeling," Vunjak-Novakovic adds. "In all these diverse areas, we now can put the cells to work for us and make tissues, by providing bioengineered environments that mimic their native milieu."
Original publication
Bhumiratana, Sarindr and Bernhard, Jonathan C. and Alfi, David M. and Yeager, Keith and Eton, Ryan E. and Bova, Jonathan and Shah, Forum and Gimble, Jeffrey M. and Lopez, Mandi J. and Eisig, Sidney B. and Vunjak-Novakovic, Gordana; "Tissue-engineered autologous grafts for facial bone reconstruction"; Science Translational Medicine; 2016






















































