When the reflection kills
A mirrored amino acid specifically inhibits the growth of certain cancer cells
Advertisement
In nature, there are often substances that behave like mirror images, such as the well-known left- and right-turning lactic acid. Research teams from the University of Marburg and the University of Geneva have now discovered how the growth of certain cancer cells can be specifically weakened with a mirror image substance - namely with the amino acid D-cysteine, which is an exact mirror image of the natural, sulphur-containing amino acid L-cysteine. The researchers, led by Prof. Roland Lill from the Institute of Cytobiology at Philipps University Marburg, report on this in Nature Metabolism.
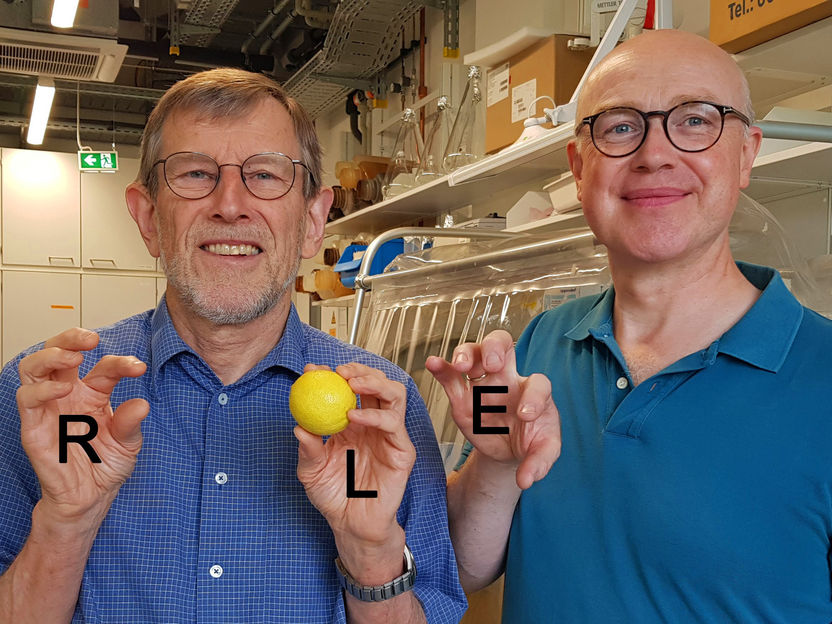
In the illustration, Roland Lill (left) and Oliver Stehling show an example of how D-cysteine has a toxic effect in cells: Normally, the yellow sulphur is transferred from the left hand (L) to a receptor position in the enzyme (E). However, if the yellow sulphur is located in the right hand (R), the distance to the receiver position is too great. As a result, the transfer reaction that is vital for the cell cannot take place and the cell dies.
Foto: Carina Beimborn; Bearbeitung: Roland Lill
In contrast to L-cysteine, however, the mirror image D-cysteine cannot be used to synthesize protein molecules (proteins) in biological cells. The research teams have now worked out the exact mechanisms by which D-cysteine inhibits the growth of tumor cells. Initially, Prof. Jean-Claude Martinou's group in Geneva observed that only those tumor cells in which an already known cysteine transporter was increasingly present in the cell membrane were inhibited in their growth by D-cysteine. This was indeed the case in most of the tumor cells tested, as this transporter actually provides the tumor cells with growth advantages through the uptake of L-cysteine.
The Marburg laboratory headed by Roland Lill, who has long been involved in the process of the biological production of so-called iron-sulphur proteins, set out to find out why D-cysteine has a toxic effect once it has been absorbed into the cell. The laboratory found that D-cysteine blocks an important enzyme that normally incorporates the sulphur of L-cysteine into iron-sulphur proteins so that they can work in the cell. Since many iron-sulfur proteins perform vital functions, such as DNA synthesis, a cell cannot function properly without these iron-sulfur proteins and therefore perishes.
The researchers were able to show in detail how D-cysteine blocks the sulphur-releasing enzyme. In simple terms, the natural L-cysteine transfers its sulphur to the enzyme with its "left hand". In the mirror image D-cysteine, however, the sulphur is in the "right hand" and therefore too far away from the receiver position in the enzyme. The transfer of the sulphur is therefore blocked and, as a result, no iron-sulphur proteins can be formed.
"The investigations could have relevance for tumor therapy," comments Lill. Initial experiments on mice in the Geneva laboratory have shown that D-cysteine can also significantly inhibit the growth of tumors in living animals. The researchers now want to test whether and how the substance can be used to treat cancer.
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.

















































