Researchers discover previously unknown "mini-factories" for protein folding
Advertisement
In order for proteins to perform their many functions, they must be folded correctly. A research team at the University of Basel has now discovered a type of "folding factory" in which proteins are folded efficiently and without errors. If these factories are missing, this can lead to diseases such as diabetes or neurodegenerative disorders.
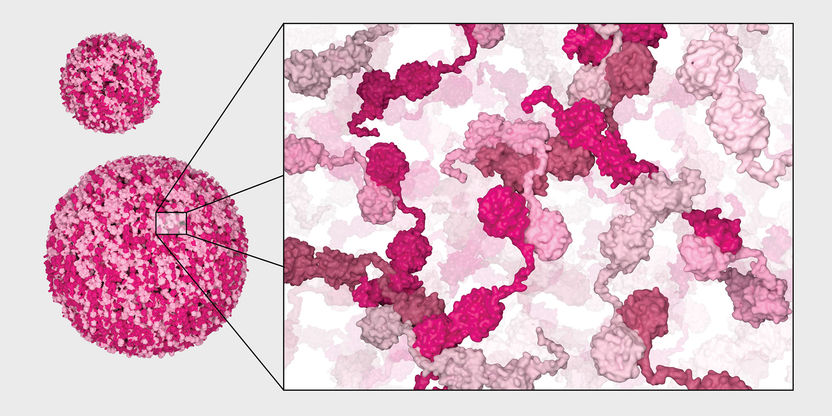
Model of the formation of chaperone condensates.
Copyright: Biozentrum, Universität Basel
Proteins are the workers in our cells: They transport substances, help with digestion and serve as building materials. In order to be able to perform their many tasks, they must be brought into the correct three-dimensional structure, i.e. folded correctly. This is ensured by a whole arsenal of folding helpers: the chaperones. Together with Prof. Dr. Anne Spang, Prof. Dr. Sebastian Hiller's team at the Biozentrum, University of Basel, has now been able to show for the first time that chaperones independently assemble into "folding factories". This is where the newly produced, initially unfolded proteins are brought into the correct form.
The starting point for the work was a clinical observation: mutations in a specific chaperone, PDIA6 for short, were found in several families with genetic diseases, including liver fibrosis, diabetes and cognitive impairment. "This observation sparked our interest," says Hiller. "We asked ourselves what PDIA6 is actually important for and therefore began to investigate its function in the cell."
"Folding factory" in the cell
The endoplasmic reticulum (ER) is the structure inside cells in which proteins are folded. This is why there are numerous chaperones there. "Traditionally, it was thought that the folding helpers swim around individually in the ER," says Anna Leder, first author of the paper now published in "Nature Cell Biology". "However, we discovered that they organize themselves independently and form droplet-like structures, so-called condensates."
These condensates correspond to a conveyor belt on which the machines for protein folding are optimally arranged. PDIA6 ensures that several different chaperones join together. PDIA6 molecules interact with each other and initiate the formation of a condensate, into which numerous other chaperones join. "The concentration of folding helpers is very high at this site, so that unfolded or misfolded proteins are literally pulled into it," explains Leder. "Only when they are correctly folded are they released again." The condensates are used for quality control and also increase the efficiency of protein folding.
No insulin without condensates
But what happens if these folding factories are missing? The cells are then massively stressed and, in the worst case, they die because too many unfolded or incorrectly folded proteins remain. This is exactly what the researchers were able to observe in further experiments.
"We looked at the hormone insulin, which regulates blood sugar," says Leder. "The precursor pro-insulin is only folded correctly within the condensates. In cells with mutations in the PDIA6 chaperone, these condensates do not form. They therefore produce less insulin and also secrete less of it." This is consistent with clinical observations in which patients with PDIA6 mutations suffer from diabetes, among other things.
More than the sum of its parts
"This discovery is a real game changer," says Hiller. Such chaperone condensates were previously unknown. They are not just an incidental side effect, but an important organizational unit. "We may have to thoroughly rethink the concept of the ER, and possibly other cell organelles as well," says Hiller. "We can probably only explain and really understand how the ER works with the presence of condensates."
Knowledge of the self-organization of chaperones is important for further research and, in the long term, for medical practice. Numerous diseases are linked to incorrectly folded proteins, such as neurodegenerative diseases, diabetes, cancer and cystic fibrosis.
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.