celares GmbH brings GMP-production facility into operation
Advertisement
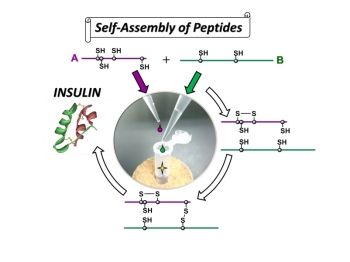
The Berlin drug delivery company celares GmbH has brought a GMP production facility for the synthesis of activated PEG-reagents into operation in September. The company now belongs to a handful of worldwide suppliers that is capable of producing these special reagents in the required quality for pharmaceutical applications.
Activated PEG-reagents are important start materials for the manufacturing of PEGylated drugs. By PEGylation the pharmacological properties of drugs are improved and their tolerance by the patient is increased. At its location Berlin-Buch celares GmbH has invested more than one million Euro by own means in the construction of clean rooms and the installation of equipment. Core is a modern 60L reactor for the actual chemical synthesis of the PEG-reagents in a multi-kilogram scale. The complete process is performed according to the current guidelines for Good Manufacturing Praxis (cGMP). In this way the use of these products in the pharmaceutical field is possible.
In addition to the distribution of PEG-reagents for scientific applications, customised development services for pharmaceutical companies have been the main business of celares GmbH so far. Since 2003 the company has successfully developed several PEGylated drugs up to the beginning of clinical development. With the installation of a production facility celares GmbH can now provide PEG-reagents for the full clinical development phase and market supply to existing and new clients. The company expects a significant increase in turn over by this activity and will grow to 25 employees within the next months.
Most read news
Organizations
Other news from the department manufacturing
These products might interest you

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.