Cell to cell efficiency in the brain
Advertisement
A new molecular mechanism behind certain learning and memory processes is revealed in Neuron. An international team show how neuronal activity can change synaptic efficacy in the brain and how this is modified by type-A GABA receptors.
With their discovery, the research group from RIKEN, Japan Science and Technology Agency, Japan, Ecole Normale Supérieure Paris and Institut National de la Santé et de la Recherche Médicale U789, France, has overturned conventional thinking on the regulatory mechanism of synaptic transmission efficiency.
The efficiency with which information is transmitted between nerve cells is central to the neurochemical foundations of memory and learning. Researchers know that one of the factors determining the efficiency of neurotransmission is the number of neurotransmitter receptors in the junction through which signals pass between nerve cells – known as the synapse.
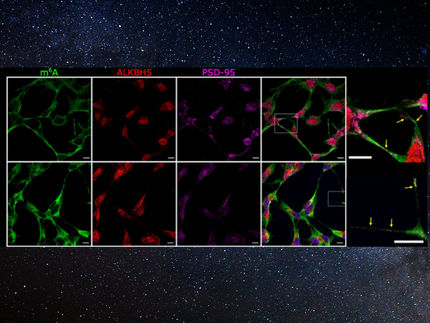
By tracking single particle subunits of one neurotransmitter receptor – the type-A GABA receptor (GABAAR) – in cultured hippocampal neurons, the team shows that it diffuses in two dimensions, and its diffusive behavior changes in parallel with neuronal activity. They then determined that such lateral diffusion is regulated by the activation of calcineurin, which results from increases of calcium concentration in the cell.
An activity-dependent change in synaptic efficacy is a central tenet in learning, memory, and pathological states of neuronal excitability. The lateral diffusion dynamics of neurotransmitter receptors are one of the important parameters regulating synaptic efficacy.
Lateral diffusion, regulated through receptor-scaffold interactions, provides a simple mechanism for rapid and reversible activity dependent modulation of synaptic strength. Defective regulation may also be involved in pathological conditions such as status epilepticus - a life-threatening condition in which the brain is in a state of persistent seizure.
“Elucidating the details of the molecular mechanisms controlling receptor diffusion dynamics would help us not only to understand a basic physiological mechanism that may be involved in learning and memory, but also to define new targets for pharmacological approaches to critical neuronal diseases such as epileptic seizures,” says a member of the research team, Hiroko Bannai of the RIKEN Brain Science Institute.
Original publication: Bannai, H., Lévi, S., Schweizer C., Inoue, T., Launey T., Racine V., Sibarita, JB., Mikoshiba K., Triller A.; "Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics."; Neuron 62(5) (2009).