Micro-Dx™ CE IVD
Unique Automated Pathogen DNA Extraction and Broad-Range Molecular Diagnosis of Bacteria and Fungi


Walk-away selective microbial DNA extraction, Real-Time PCR detection and sequence identification
Micro-Dx™ CE IVD is a rapid test for the culture-independent identification of pathogens
Micro-Dx™ CE IVD for the first time runs a test system that is based on the fully automated MolYsis™ technology to remove human DNA from clinical specimens and extraction of enriched pathogen DNA. This together with the extremely active broad-range PCR or Real-Time PCR followed by sequencing analysis enables the sensitive detection and identification of pathogens. All buffers, reagents and consumables are DNA-free which eliminates false-positive results due to endogenous contamination.
Walk-away automation of pathogen DNA extraction
Micro-Dx™ CE IVD is the premiere of a walk-away automation in sample preparation for the precise, direct diagnosis of pathogens. Micro-Dx™ CE IVD follows the MolYsis™ technology of the removal of human DNA, which is a known factor negatively influencing PCR analysis. The whole automation process of ‘Sample in – Eluate-out’ greatly reduces tedious, time-consuming extraction. Handling is reduced to the loading of the SelectNA™plus instrument with 1ml samples and consumables. The instrument can run 1 to 12 samples simultaneously.
Typical specimens:
- Body fluids – EDTA/citrate blood, CSF, synovial fluid, ascites fluid and others
- Tissue biopsies – heart valve, bone, brain, liver and others
- Other specimens – BAL, Smears, swabs, wound biopsies
Broad-range molecular analysis
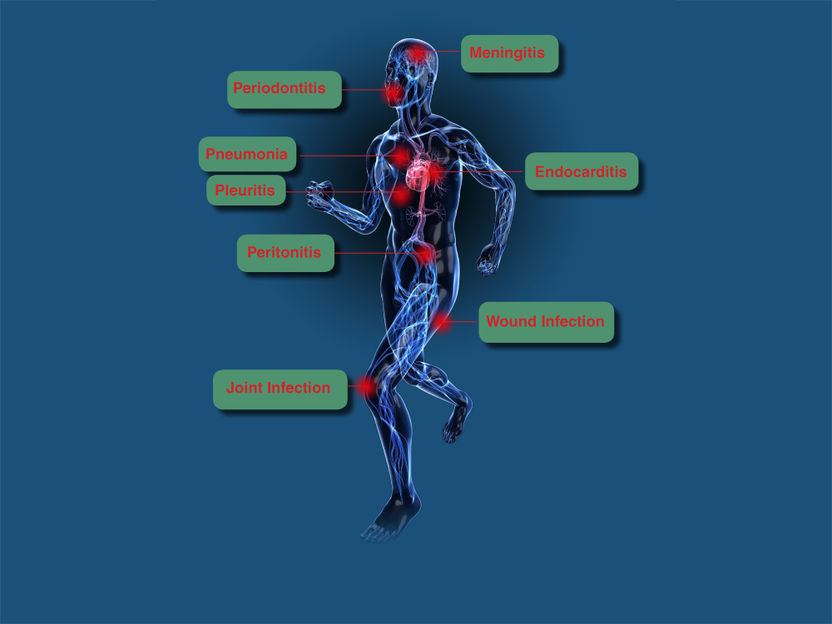
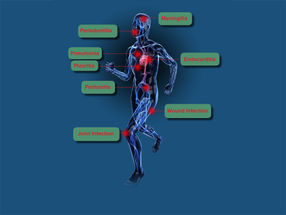
Micro-Dx™ CE IVD can diagnose typical, rare and slow-growing pathogens. The Micro-Dx™ CE IVD diagnosis is growth-independent and thus particularly useful for the identification of non-growing pathogens in patients who are on antibiotic therapy. Clinical studies indicate that Micro-Dx™ CE IVD finds pathogens in many cases where cultures stay negative. The diagnosis is directed to diseases including:
- Sepsis
- Meningitis
- Wound infections
- Joint infections
- Endocarditis and others
Guaranteed DNA-free reagents and consumables are supplied necessary for the broad range 16S and 18S rRNA gene PCR or Real-Time PCR analysis together with primers for Sanger-sequencing based identification of pathogens in positive cases. Micro-Dx™ CE IVD serves a variety of specified PCR and Real-Time PCR run protocols.
Sequence identification of pathogens is performed using the free online tool, SepsiTest-Blast (www.sepsitest-blast.net) with >7,000 sequence-edited entries of cultured bacterial, Candida, Cryptococcus and Aspergillus species.
For more detailed information, including a list of publications, a quotation or presentation in your laboratory, please feel free to contact the Molzym support-team.

1
SelectNA™plus Instrument

2
Micro-Dx™ CE IVD – Diagnosis of Infectious Diseases

3
Micro-Dx™ CE IVD – Diagnosis of Infectious Diseases
Request information about Micro-Dx™ CE IVD now

DNA extraction kits: Micro-Dx™ CE IVD
Unique Automated Pathogen DNA Extraction and Broad-Range Molecular Diagnosis of Bacteria and Fungi
Product classification Micro-Dx™ CE IVD
Product categories
Applications
Manufacturers of similar products
Advertisement
Find more DNA extraction kits and related products
Find Micro-Dx™ CE IVD and related products in the theme worlds
Topic World PCR
This groundbreaking and highly versatile molecular technique of PCR allows us to amplify tiny amounts of genetic material on a large scale and analyze them in detail. Whether in medical diagnostics, forensic DNA analysis or research into genetic diseases - PCR is an indispensable tool that gives us deep insights into the world of DNA. Immerse yourself in the fascinating world of the polymerase chain reaction (PCR)!

Topic World PCR
This groundbreaking and highly versatile molecular technique of PCR allows us to amplify tiny amounts of genetic material on a large scale and analyze them in detail. Whether in medical diagnostics, forensic DNA analysis or research into genetic diseases - PCR is an indispensable tool that gives us deep insights into the world of DNA. Immerse yourself in the fascinating world of the polymerase chain reaction (PCR)!