Crucell Doubles Production of Quinvaxem in 2008 and Prepares Move to New Korean Production Site to Facilitate Growth
Crucell N.V. announced that an agreement was reached to relocate Crucell's Korean production facility, where its pentavalent children's vaccine Quinvaxem® and a hepatitis B vaccine Hepavax-Gene® are produced, from the Shingal site in Yongin City, Korea to the Incheon Free Economic Zone.
All parties involved have agreed on the time line and conditions of this relocation, enabling a smooth transition to the new production facility. With engineering well underway, construction activities at the new site are due to start soon. The new facility will enable the further growth and efficient production of Quinvaxem® and Hepavax-Gene®. No further details were provided.
"We are pleased that we were able to settle all litigation around our production facility amicably, on terms beneficiary to all parties. Production of Quinvaxem® at our existing site in Shingal has already doubled compared to last year, and the new production facility will support the expected growth of our flagship products in the years to come." said Cees de Jong, Chief Operating Officer of Crucell.
Most read news
Other news from the department manufacturing

Get the life science industry in your inbox
From now on, don't miss a thing: Our newsletter for biotechnology, pharma and life sciences brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.
Most read news
More news from our other portals
Last viewed contents
Health_insurance
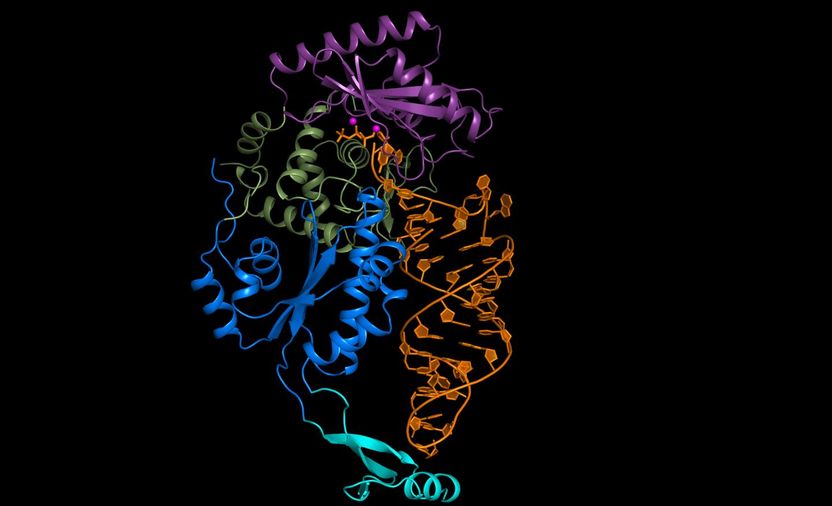
In a role reversal, RNAs proofread themselves - Molecular photographs of an enzyme bound to RNA reveal a new, inherent quality control mechanism

HLC - Haep Labor Consult - Bovenden, Germany
Royal_College_of_Physicians
























































