Remote-controlled implantable device delivers HIV prevention drug
Advertisement
A Houston Methodist research team received a nearly $4 million grant to test a transcutaneously refillable implant that administers pre-exposure prophylaxis drugs to subjects at risk of HIV-exposure.
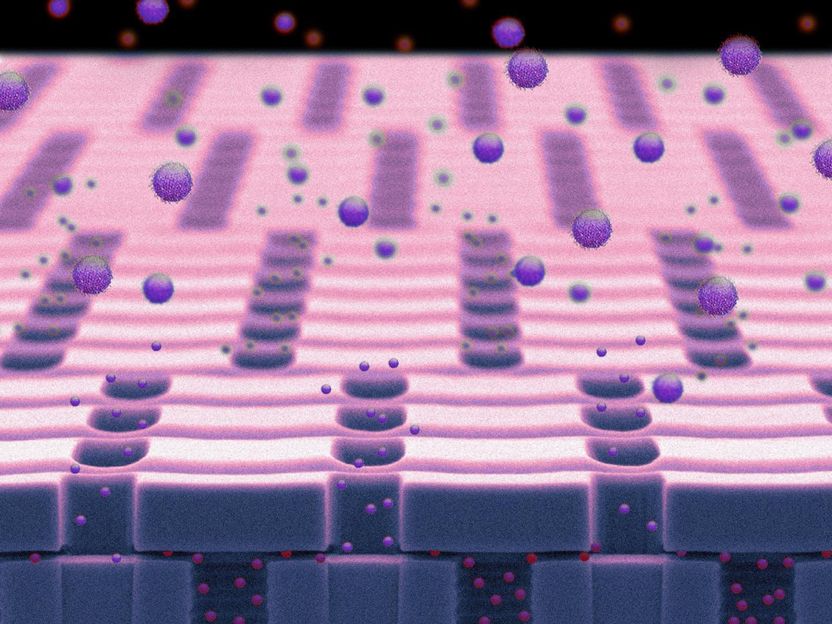
A nanochannel membrane is shown within the nanochannel delivery system (nDS)
Houston Methodist
The National Institute of Allergy and Infectious Diseases (NIAID) awarded Alessandro Grattoni, Ph.D. , the multi-million dollar grant over five years to enhance the nanochannel delivery system (nDS), which provides sustained and constant release of drugs without the use of pumps, valves or a power supply.
Grattoni, chair of the Department of Nanomedicine at Houston Methodist Research Institute , leads the project which has already shown in pilot studies that the device successfully released tenofovir alafenamide as a preventive, pre-exposure prophylaxis over 21 days in animal models. The NIAID-funded research will aim for larger nanochannels in the nDS to allow for a 60-day drug delivery.
Grattoni, also director of the Center of Space Nanomedicine at Houston Methodist , said the device will be tested during three of 10 research projects planned aboard the International Space Station over the next five years.
Many high-risk patients already take Truvada, a combination therapy of tenofovir disoproxil fumarate and emtricitabine to help prevent HIV-1 infection. However, the Centers for Disease Control says poor patient adherence is an ongoing challenge. Grattoni's implantable device achieves sustained drug delivery by controlling diffusion through nanochannel membranes engineered to be close to the size of the drug molecules being released. The nDS is implanted just under the skin and refilled through a port as needed. If successful, the research will next move into patient clinical trials.
The World Health Organization estimated nearly 37 million people worldwide living with HIV/AIDS, and approximately 2 million newly-infected people worldwide, in 2014.
In addition to the HIV-prevention drugs, Grattoni and team have tested more than 50 drugs in the device. The broader goal is to prove the technology is flexible and applicable to a variety of therapies including hormone replacement, cancer prevention and treatment, mental disorders, drug abuse and metabolic syndrome.
















































