New drug combination restores beta cell function in animal model
Potential for diabetes remission
Advertisement
The loss of the identity of insulin-secreting beta cells in the islet of Langerhans, a process also called beta cell dedifferentiation, has been proposed to be a main reason for the development of diabetes. If and how dedifferentiated beta cells can be targeted by pharmacological intervention for beta cell regeneration is unknown. In a new study in mice, Helmholtz Zentrum München in collaboration with Novo Nordisk, demonstrated for the first time that a targeted combinatorial drug treatment is able to restore beta cell function, achieve beta cell redifferentiation and therefore potentially open new ways for diabetes remission.
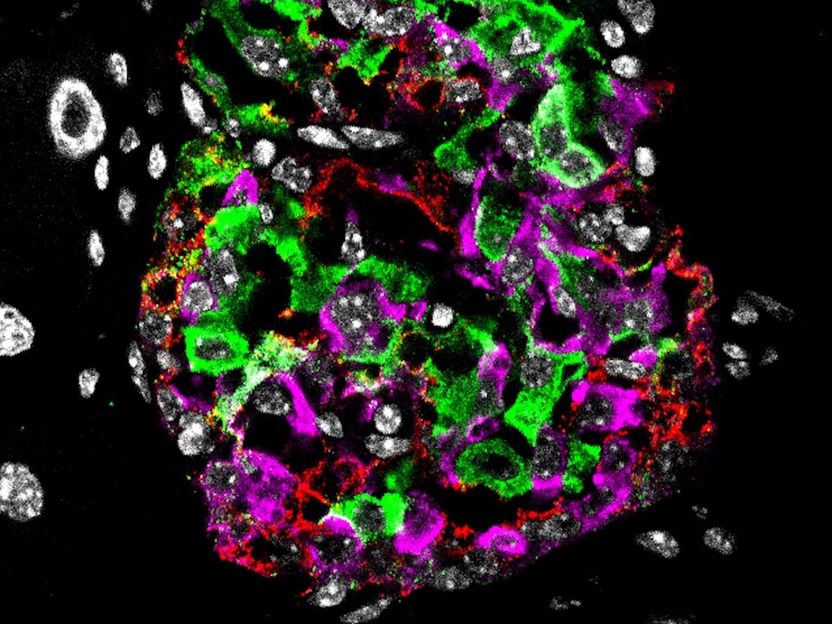
Pancreatic islet from a diabetic mouse: Cell nucleus in white, beta cells and insulin in green, alpha cells (hormone glucagon) in red and delta cells (hormone somatostatin) in magenta.
Helmholtz Zentrum München / Aimée Bastidas-Ponce
Under certain conditions, beta cells can lose their identity and regress to a less differentiated state in which they lose most of their prior functions. It has been proposed that this dedifferentiation contributes to an ongoing degenerative process of beta cell dysfunction. Current pharmacological diabetes treatments are not able to stop the decline of functional beta cell mass loss. The earlier this decline can be prevented, ideally already when first diabetic symptoms appear, the higher the amount and the level of beta cell function that will be preserved.
New target: Dedifferentiated beta cells
To investigate whether dedifferentiated beta cells can be targeted pharmacologically to restore beta cell function, the researchers used streptozotocin-induced diabetes in mice. Streptozotocin kills insulin-producing beta cells and causes severe diabetes. When injected in multiple low doses, however, some beta cells survive which replicates the decline in function the researchers wanted to establish for their experiment. Using single cell RNA sequencing the researchers could show that after streptozotocin treatment, the surviving beta cells dedifferentiate into a dysfunctional state. The simplicity of the model used (no genetic lesions nor autoimmunity) would help them better monitor the effect of the pharmacological treatment.
A good match: GLP-1/estrogen and insulin have additive effects in preclinical models
The team then tested treatments for their potential to restore beta cell function. To this end, they stratified seven cohorts of severely diabetic mice and treated them daily for 100 days with single and combinatorial pharmacology. The researchers showed that a stable Glucagon-like peptide-1 (GLP-1)/estrogen conjugate (provided by Novo Nordisk) enables targeted and selective delivery of the nuclear hormone cargo to beta cells. The combination of GLP-1/estrogen and a long acting insulin was superior to mono-treatments to both normalize glycemia, glucose tolerance, to increase pancreatic insulin content and to increase the number of beta cells. Importantly, administration of high doses of GLP-1/estrogen did not show signs of systemic toxicity in rats, a pre-requisite for any future clinical testing. In a collaboration with the biotech company InSphero, the researchers could also show that GLP-1/estrogen, but not GLP-1 or estrogen alone, increases human beta cell function when human pancreatic islets are exposed to cytokine stress, which is known to impair human beta cell function.
“Not only does our study describe paths and processes of beta cell dedifferentiation, it also demonstrates the potential of single and combinatorial drug treatments to achieve diabetes remission by targeting dedifferentiated beta cells” explains Prof. Dr. Heiko Lickert, director of the Institute for Diabetes and Regeneration Research at Helmholtz Zentrum München and Professor of Beta Cell Biology at TUM School of Medicine. “This is the first study that shows beta cell redifferentiation with targeted pharmacology performed by an interdisciplinary team that used cutting edge single cell technology, computational biology, pharmacology and regeneration biology“ says Lickert who, together with Susanna M. Hofmann, Fabian Theis and Timo D. Müller at Helmholtz Zentrum München, lead this research project.
Future clinical studies?
This study brought together scientists from Helmholtz Zentrum München (Helmholtz Diabetes Center and Institute for Computational Biology), the German Center for Diabetes Research (DZD), Technical University Munich (TUM) as well as InSphero AG and Novo Nordisk with the aim to explore the potential therapeutic benefits of GLP1/estrogen treatment in an animal models and in human cells in vitro. The results from this study as well as future studies to support translation to humans and safety of the compound may pave the way for clinical studies that use GLP-1 as a carrier peptide for estrogen but potentially also other, novel cargos to directly target beta cells for regenerative therapy and diabetes remission.























































