Gene therapy triggers growth of new auditory hair cells in mammals
Advertisement
University of Michigan scientists have used gene therapy to grow new auditory hair cells in adult Guinea pigs - a discovery that could lead to new treatments for human deafness and age-related hearing loss.
Healthy hair cells are vital to the ability to hear, but aging, infection, certain medications and exposure to loud noises can damage or destroy hair cells causing sensorineural hearing loss - a condition affecting over 30 million Americans. Since the discovery, in the late 1980s, that birds can spontaneously regenerate damaged hair cells, scientists have been trying to find a way to induce the replacement of lost hair cells in mammals.
U-M scientists have now accomplished this goal by inserting a gene called Math1 into non-sensory epithelial cells lining the inner ear. Results from the study will be published in the June 1 issue of the Journal of Neuroscience.
"We found that non-sensory epithelial cells in adult guinea pig cochlea can generate new sensory hair cells following the expression of Math1," says Yehoash Raphael, Ph.D., an associate professor of otolaryngology in the U-M Medical School, who directed the study. "We also found that some of these hair cells can attract the growth of new fibers from auditory neurons."
In a normal ear, vibrations from sound waves striking the eardrum are transferred to fluid inside a snail-shaped bony organ called the cochlea, which is the auditory component of the inner ear. When cochlear fluid moves, it stimulates movement in thousands of tiny projections on hair cells lining the inside of the cochlea. Moving hair cells initiate electrical signals, which are picked up by auditory nerve fibers and carried to an area of the brain called the auditory cortex. If hair cells are damaged or missing, electrical signals are not generated and hearing is impaired.
"During the embryonic stage of an animal's development, hair cells and supporting cells have a common origin. Cells that express Math1 are fated to become hair cells, while Math1 expression is inhibited in the remaining non-sensory cells," Raphael says.
"After embryonic development, hair cell production ceases. Unlike other epithelial cells in the skin or gut, epithelia in the inner ear contain no stem cells, so there is no source for renewal," Raphael explains. "That's the main reason why hair cell loss is permanent. When we over-expressed Math1 in non-sensory cells of the mature cochlea, however, we found that it causes them to transdifferentiate or change their personality to become hair cells."
"We knew that transdifferentiation of supporting cells was a major source of new hair cell development in birds," Raphael says. "But there was no proof it would work in mammals. We started gene therapy experiments in 1994 and it took us seven years to develop a successful method of introducing the gene into the non-sensory cochlear epithelium."
Dr. Kohei Kawamoto, Ph.D., a former U-M research fellow who performed the laboratory experiments, used an adenovirus as a vector to deliver the Math1 gene to inner ear epithelial cells. Kawamoto injected the Math1 vector into inner ear fluid of 14 adult guinea pigs. The same procedure, but without the transfer of the Math1 gene, was performed on 12 matched control animals.
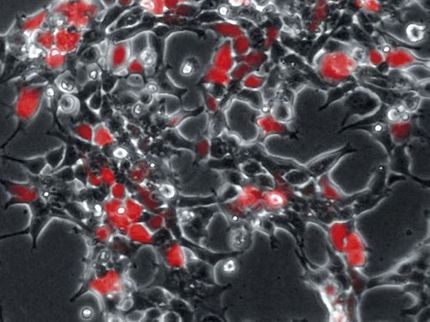
Thirty to 60 days after inoculation, U-M scientists used scanning electron microscopes to examine inner ears from both sets of animals. In experimental guinea pigs that received the Math1 gene, scientists found new hair cells growing in areas where hair cells are typically absent. No new hair cells were found in the control animals.
"The inner ear is an ideal target for gene therapy, because it is closed - not sealed, but nicely isolated," Raphael says. "As long as the amount you inoculate is small, the spread to other organs is minimal, and the risk of systemic toxicity is almost zero."
Because the total amount of fluid in the inner ear of a guinea pig is so small, the mechanical impact of injecting the viral vector fluid into the cochlear fluid damaged some of the hair cells in experimental animals. "While this is a concern, we believe the micro-injection technology can be improved to prevent this mechanical trauma," Raphael says. "The human cochlea is larger than a guinea pig cochlea and may better tolerate the inoculation. Also, profoundly deaf human candidates for this gene transfer approach would likely have severe pre-existing hair cell loss to begin with, so the risk of mechanically-induced side effects would be somewhat less troubling."
One of the most surprising results of the study was the discovery of long, slender nerve fibers growing toward some of the newly formed hair cells. "This suggests that these hair cells can provide signals to attract axons and that neurons can respond to these signals," Raphael says.
In the next stage of research, Raphael will determine whether the guinea pig hair cells are functional and able to transmit sound signals to auditory neurons. He also plans to test the procedure in aging animals and in animals that are completely deaf.
"This is just the beginning," Raphael says. "It is really just a proof of the principle to show that, with proper gene therapy, these non-sensory cells have the competence to become hair cells."
Other news from the department
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Gene therapy
Genetic diseases once considered untreatable are now at the center of innovative therapeutic approaches. Research and development of gene therapies in biotech and pharma aim to directly correct or replace defective or missing genes to combat disease at the molecular level. This revolutionary approach promises not only to treat symptoms, but to eliminate the cause of the disease itself.

Topic world Gene therapy
Genetic diseases once considered untreatable are now at the center of innovative therapeutic approaches. Research and development of gene therapies in biotech and pharma aim to directly correct or replace defective or missing genes to combat disease at the molecular level. This revolutionary approach promises not only to treat symptoms, but to eliminate the cause of the disease itself.