DNA: in telomeres lesions of time are inevitable
Advertisement
A study conducted at IFOM in Milan has revealed that there are regions of the genome that are completely undefended. They are the telomeres, or extremities of the chromosomes that, by shortening with each cellular proliferation cycle, mark the inexorable passage of time and lead to cellular aging. A discovery by the team of scientists led by Fabrizio d’Adda di Fagagna at IFOM now clarifies how these cellular clocks are irreparable, independently of their shortening. In other zones of the genome efficient repair systems go to work when needed, but not here. Here the signs remain of any damage that the cell may suffer to its DNA during the course of its life. Here lesions accumulate during the aging of the organism, its tissues and cells. But if, on one hand, senescence marks the deterioration of an entire series of vital functions, on the other hand, at the cellular level, it is also a mechanism that can prevent the onset of tumours when activated precociously. The details of this research have been published in Nature Cell Biology.
DNA is the only molecule in the cell that cannot be replaced if damaged. This is not the case for other cellular constituents. For example, if a protein is somehow compromised during its functioning, as happens very frequently, the cell degrades it and replaces it with an identical one. The process starts from DNA, which is transcribed into RNA, and this in turn is translated into protein.
This does not apply to the "molecule of life", which contains the design for cellular activities and the development plan. In fact, if the DNA becomes compromised, the cell cannot follow the same intervention procedure because the template itself is ruined. In that case, the only solution for preserving the information contained in the DNA is to repair it.
However, the genome is not all repaired in the same way. The discovery that emerges in the study published today in Nature Cell Biology, conducted by Marzia Fumagalli and Francesca Rossiello under the guidance of Fabrizio d’Adda di Fagagna – director of the research program entitled “Telomeres and Senescence” at IFOM (Istituto FIRC di Oncologia Molecolare), essentially draws a map of the least defended regions of the genome: the chromosome ends, where DNA damage is irreparable.
The research, conducted in collaboration with scholars at the University of Milan-Bicocca and the New Jersey Medical School in the US, demonstrates also that the vulnerability of these parts of the genome has implications for one of the fundamental and inexorable physiological processes: Aging.
Like tissues and organisms, cells also age. To a cell that continuously divides, aging means first of all ceasing to proliferate.
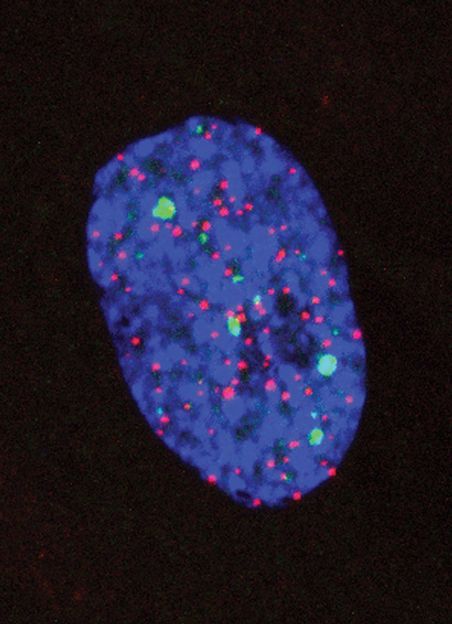
Telomeres lesions.
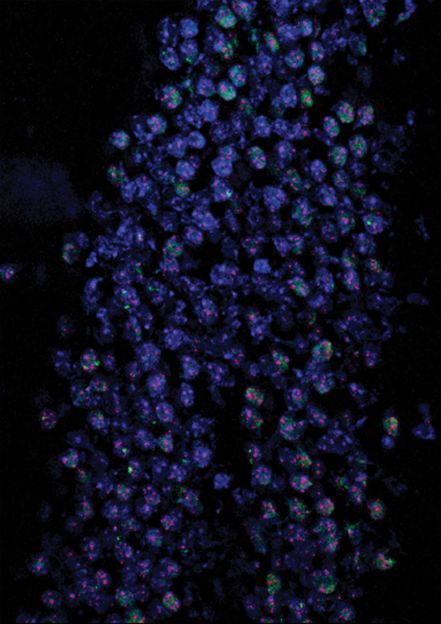
Telomeres lesions.
Proliferating cells can sense the passing of time through their telomeres; at every cycle they lose a piece until, at a certain point, they stop reproducing. This completely normal phenomenon depends on the very mechanism by which the genetic material is duplicated.
However, there are cells that do not proliferate and therefore not lose their telomeric sequences. An organism has many of these: for example, in the process of specialising to carry ou
t their functions, neurons stop dividing. How can they know that they are aging?
The answer could emerge from the implications of the discovery by d’Adda di Fagagna and collaborators.
In fact, shortening is not the only thing that can happen to telomeres with the passing of time. «Given that DNA lesions are repaired throughout the genome, except - the researchers affirm - in the telomeres, we wondered if this could be linked to aging. We found that there is a progressive accumulation of damage to these parts of the chromosomes with aging, independent of their shortening».
Therefore, cells read the passing of time not only in the length of their telomeres, but also in terms of compromised telomere integrity, a fundamental parameter in particular for cells that have ceased to divide, and therefore do not shorten their telomeres, but nevertheless continue to age.
DNA under attack
«DNA breakage is an event that is anything but rare in the life of a cell» explains d’Adda di Fagagna. «On the contrary – the scientist continues – you could say that genetic material is under practically continuous attack. Without considering extraordinary events such as exposure to radiation or various chemical and physical agents that can damage DNA, the threats come from the vital cellular activities themselves. Some examples? Respiration means also producing reactive oxygen species, so-called free radicals, that can break the DNA double helix. Copying and decoding the DNA into protein involves continuous rearrangement of the molecule: torsions, tensions and other physical stresses that accumulate in it may lead to its degeneration».
Therefore, breakage is one of the risks of living, but the researcher specifies: «As the DNA is repeatedly damaged by physiological and other events, it is also constantly repaired. Cells respond to the presence of lesions by activating a series of molecular alarms, proteins that detect damaged DNA and trigger a cascade of reactions that essentially resolve the problem. This formidable protection system is the basis of a cell’s genomic integrity and its destiny as normal or tumoural. By carefully observing cells after damaging events, however, we noticed that in some parts of the genome the characteristic alarms remained activated, but the lesions were not repaired.
Using advanced genomic technologies allowed us to examine all regions of the genome and capture those regions that were emitting persistent alarms so that we could then read their sequences. «This allowed us to locate the implicated zones, and to discover that DNA damage remained unresolved at the chromosomes ends» affirm the researchers Marzia Fumagalli and Francesca Rossiello who carried out most of the experiments, coordinated by d’Adda di Fagagna, in the laboratories at the IFOM.
If repairing broken DNA is so important to the cell, why do irreparable regions exist? Why hasn’t evolution preserved them?
This is also what the team of scientists directed by d’Adda di Fagagna asked; interpreting their data they hypothesised that: «because repair consists of putting together or merging separate DNA ends, if these ends are internal parts of a chromosome that have separated because of a break, then the repair event is a fundamental benefit for the survival of the cell itself; if instead, the DNA ends to be reunited were mistaken for the terminal parts of the chromosomes, there would be an anomalous fusion between chromosomes, something undesirable for the cell, that would put the stability and organisation of the entire genome at risk. Because of this, the ends of chromosomes are DNA elements that have evolved to prevent different chromosomes from joining to form aberrant structures. Telomeres prevent fusion/repair events by prohibiting the specific procedures that are constantly active in the rest of the genome and they do this not only at the tips of the chromosomes, but throughout its length. Therefore, irreparability of damage, is the price they pay to avoid running the risk of merging.
Cellular aging and cancer
Cellular senescence is also connected to cancer.
As we know, tumours are diseases caused by the frenzied growth of cells “gone mad”, out of control, rendered frenetic by the accumulation of multiple alterations to their genetic material. It has long been known that events linked to the origin of cancer include mutations that abnormally activate oncogenes, that is, genes that accelerate cell proliferation associated to tumoral transformation and are capable, among other things, of inducing a great quantity of DNA damage. In a vicious cycle, this damage, progressively puts most of the cellular defence and surveillance systems out of operation.
The cell has a system to prevent all of this, as emerged for the first time from a study conducted by the team of Fabrizio d’Adda di Fagagna in 2006: Precociously evoking senescence that, by blocking cellular proliferation, blocks expansion of the cells that have gone mad when an oncogene has been activated in some way.
Generally, when this fails and the cells escape the blockade imposed by the senescence mechanism, a tumour destiny becomes inevitable.
Considering this connection between senescence and cancer – concludes d’Adda di Fagagna – we are pursuing research to understand if and how the irreparable damage that accumulates in telomeres is related to the action of the oncogenes when the cell attempts to contrast them.