OctoPlus expands development agreement with ESBATech on controlled release product for ophthalmic indications
Advertisement
OctoPlus N.V. announced that it has signed a contract with ESBATech, an Alcon Biomedical Research Unit, to develop a controlled release formulation for one of its proprietary biological compounds for ophthalmic applications.
The project includes process development, scale-up and manufacturing for pre-clinical studies. Financial details are not disclosed, but the project will make a material contribution to our annual revenues in 2011. In addition to Locteron®, this programme will be part of our portfolio of projects utilising our proprietary technologies.
This drug delivery technology evaluation project started in 2009. OctoPlus evaluated the feasibility of a controlled release formulation that combines a proprietary eye care ingredient of ESBATech with our drug delivery technology PolyActive®.
Most read news
Other news from the department business & finance

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Antisoma's AS1413 regimen yields high response rate and durable responses in patients with secondary AML
Development of blood diagnostics for the early detection of cancers - bioMérieux and ExonHit Therapeutics Extend their Strategic Collaboration
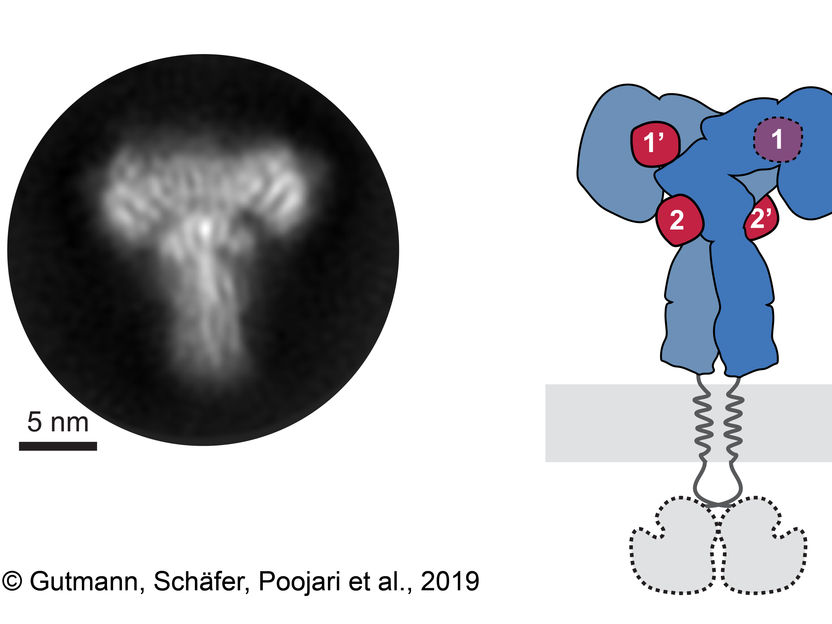
New way in which insulin interacts with its receptor discovered