How cancer puts other cells to work
Advertisement
cancer cells provide healthy neighbouring cells with additional cell powerhouses to put them to work. This has been demonstrated by researchers at ETH Zurich in a new study. In this way, cancer is exploiting a mechanism that frequently serves to repair damaged cells.
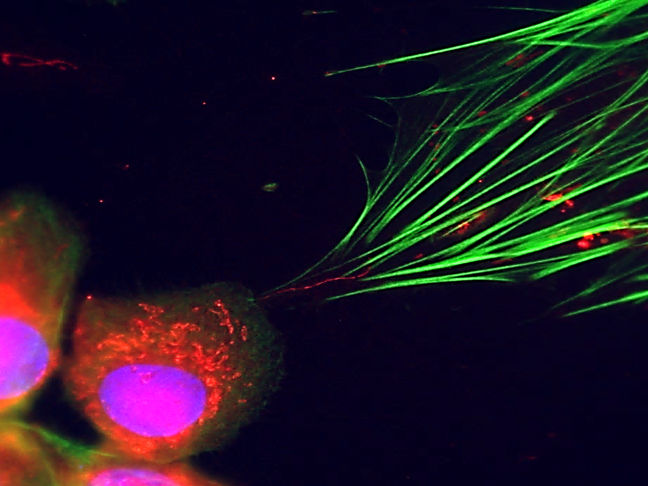
Fluorescence microscopy makes it visible: cancer cells (bottom left) can be seen transferring their cellular power plants (red dots) into connective tissue cells via small tubes (fine red line in the centre of the image). Their cytoskeleton is marked in green.
Michael Cangkrama / ETH Zürich
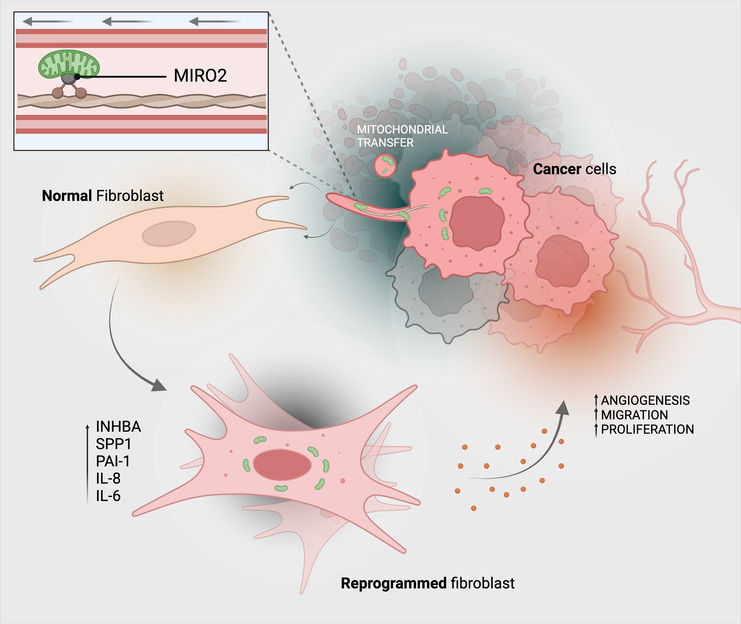
This is how cancer cells transfer their mitochondria (green) to connective tissue cells (fibroblasts).
Michael Cangkrama / ETH Zurich / BioRender

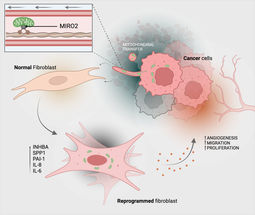
Tumours have developed many strategies and tricks to gain advantages in the body. Led by cell biology professor Sabine Werner, researchers at ETH Zurich have now discovered another surprising trick that certain tumours resort to in ensuring their survival and growth.
In a new study published in the journal Nature Cancer, the biologists show that skin cancer cells are able to transfer their mitochondria to healthy connective tissue cells (fibroblasts) in their immediate vicinity. Mitochondria are the cell compartments that provide energy in the form of the molecule ATP.
The cancer cells use tiny tubes made of cell membrane material to transfer the mitochondria and connect the two cells – much like in a pneumatic tube system.
Functional reprogramming
The mitochondrial transfer reprograms the fibroblasts functionally into tumour-associated fibroblasts, which mainly support cancer cells: tumour-associated fibroblasts usually multiply faster than normal fibroblasts and produce more ATP, while also secreting higher amounts of growth factors and cytokines. And all this benefits the tumour cells: they also multiply faster, making the tumour more aggressive.
Last, but not least, the hijacked fibroblasts also alter the cell environment – the so-called extracellular matrix – by increasing the production of certain matrix components in such a way that cancer cells thrive. The extracellular matrix is vital for the mechanical stability of tissues and influences growth, wound healing and intercellular communication.
From chance discovery to therapy?
It was actually a chance discovery, as Sabine Werner related. Her former postdoctoral researcher Michael Cangkrama discovered tiny tube-like connections between the two cell types in a Petri dish containing a co-culture of fibroblasts and skin cancer cells. He was then able to show that mitochondria from cancer cells are transferred into fibroblasts by way of these nano-connections.
The fact that cells are able to exchange mitochondria by way of such connections is nothing new in itself. For example, scientists discovered several years ago that after a stroke, healthy cells in nerve tissue pass on their powerhouse organelles to damaged nerve cells to ensure their survival. "Cancer cells actually exploit a mechanism for their own purposes that is beneficial in the event of injury. This allows them to grow into malignant tumours," as Werner explains. Other research groups have shown that cells from the tumour environment can transfer their mitochondria to cancer cells, which enhances the fitness of the recipient cancer cells. To date, however, it was not known that the mitochondrial transfer also works in reverse, from skin cancer cells to healthy connective tissue cells.
In collaboration with other research groups at ETH Zurich, the researchers found evidence that this transfer also plays a role in other cancer types, such as breast cancer and pancreatic cancer. This is particularly important in the latter case because pancreatic tumours contain many fibroblasts, and their connective tissue is relatively large.
The protein MIRO2 aids in the transfer
Finally, the researchers also clarified the molecular mechanism behind the mitochondrial transfer. Some proteins were already known to assist in transporting mitochondria. The researchers investigated which of these proteins were present in large numbers in cancer cells that transfer mitochondria and came across the protein MIRO2. "This protein is produced in very high quantities in cancer cells that transfer their mitochondria," says Werner.
The researchers detected MIRO2 not only in cell cultures, but also in samples of human tissue - especially in tumour cells at the edges of tumors that grow invasively into the tissue and occur in close proximity to fibroblasts. "We were able to detect MIRO2 exactly where we expected it to be," as first author Michael Cangkrama stated.
In search of an inhibitor
The new findings offer starting points for arresting tumour growth. When the researchers blocked the formation of MIRO2, the mitochondrial transfer was inhibited, and the fibroblasts did not develop into tumour-promoting fibroblasts.
"The MIRO2 blockade worked in the test tube and in mouse models. Whether it also works in human tissue remains to be seen," says Werner. To find this out, the researchers first need to identify an inhibitor for MIRO2 that has few side effects in the human body. "If successful, such an inhibitor could be transferred to clinical applications in the longer term." It is likely to be years, however, before such a therapy is developed and tested.
Original publication
Michael Cangkrama, Huan Liu, Xiaoyu Wu, Josephine Yates, James Whipman, Christoph G. Gäbelein, Mai Matsushita, Luca Ferrarese, Sibilla Sander, Francesc Castro-Giner, Simran Asawa, Magdalena K. Sznurkowska, Manfred Kopf, Jörn Dengjel, Valentina Boeva, Nicola Aceto, Julia A. Vorholt, Sabine Werner; "MIRO2-mediated mitochondrial transfer from cancer cells induces cancer-associated fibroblast differentiation"; Nature Cancer, 2025-8-28