A sustainable photocatalytic method to introduce the pyridine and valuable functional groups into alkenes
An excellent functional group tolerance made this protocol available for the late-stage modification of drugs
Advertisement
pyridine is one of the most important structural motifs that widely exist in abundant approved drugs and play an indispensable role in the biological activities of the drugs. Therefore, developing a sustainable method to construct pyridine-containing compounds is desirable. In the past decade, visible light catalysis has played a significant role in organic synthesis due to the mild conditions and high efficiencies for the construction of various value-added skeletons. Recently, difunctionalization of alkenes via photochemistry has attracted increasing attention and has been widely used to simultaneously introduce pyridine and other functional groups.
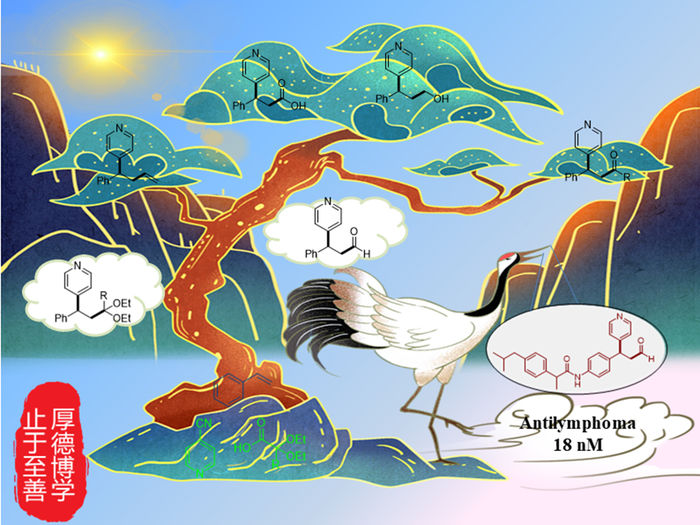
The first general transition-metal-free, photoinduced acetalation-pyridylation of alkenes using diethoxyacetic acid and cyanopyridine under mild conditions is reported. This environmentally friendly method is tolerant to many functional groups and independence of chemical oxidants. Various functional group transformation, late-stage modification of drugs, and good in vitro antitumor activity indicate this protocol is of significance and potential for drug development.
Chinese Journal of Catalysis
However, the strategies that directly installing both the pyridine group and the acetalate group into alkenes are still elusive and rare. The general method to simultaneously introduce pyridine and valuable functional groups, such as aldehyde group, keto group, hydroxymethyl group, alkenyl group, is also elusive.
Recently, a research team led by Prof. Yuqin Jiang from Henan Normal University, China and Prof. Bing Yu from Zhengzhou University, China reported the first visible light-promoted transition-metal-free acetalation-pyridylation of alkenes using 4CzIPN as a photocatalyst and glyoxylic acid acetal as a formyl equivalent. The acetal group could be easily converted into an aldehyde group, which could be further transformed into various functional groups by simple operations. The methodology provides a diverse way to hydroformylation-pyridylation, ketonization-pyridylation, hydroxymethylation-pyridylation, and alkenylation-pyridylation of styrene for the first time. The excellent functional group tolerance made this protocol available for the late-stage modification of drugs. Moreover, the good in vitro antitumor activity of the products indicates that this visible light-promoted protocol is of significance and potential for antitumor drug development.























































