Discovery of a new cancer-inducing gene and its therapeutic weaknesses
A mutation of the RRAS2 gene acts as a cancer activator in several cells of the organism, according to a study led by CSIC researchers
Advertisement
CSIC researchers lead a study that has shown that a mutation of the RRAS2 gene acts as a cancer inducer in a broad spectrum of cells in our body. Through the study of tumor cells generated after the expression of this mutant version, this work, published in Cell Reports, has also illuminated the changes it causes in each of the cell types that give rise to these tumors. This has led to the discovery of Achilles' heels in each of these tumors, which in turn has led to the identification of drugs that could be used to treat patients with tumors harboring mutations in this gene.

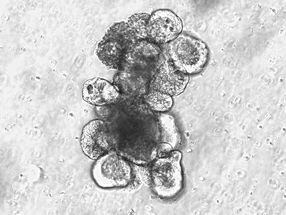
Tumor cells induced by expression of the mutant version of RRAS2.
CIC
Intensive sequencing of the tumor genome over the last few years has identified thousands of genetic alterations, called mutations. The great scientific challenge is to determine which of these mutations are relevant in the development of cancer and, after that, to discover the changes they cause in the normal cells of our organism to turn them into malignant cells. In addition, most genetic alterations present in human cancers are found at very low frequencies, making it extremely difficult to establish their relevance using only human patients. "Addressing these challenges is important not only for understanding the cause of cancer, but also for the effective implementation of personalized medicine, which is based on the design of therapies based on the pattern of mutations that tumors exhibit in specific patients," says Xosé Bustelo, coordinator of the study and CSIC researcher at the Cancer Research Center (CIC-CSIC-USAL).
The mutation that has been studied by these researchers represents a very small alteration, since it involves the change of a single letter (nucleotide) of the 82,000 of which the RRAS2 gene is composed. However, this small change is critical, since it causes the molecule encoded by this gene to change its behavior radically. "While the normal version of this molecule functions like a switch that can be turned on or off depending on the presence of various extracellular messages, the mutant version is permanently anchored in the activated state, which makes it function chronically without ever being able to turn off. This causes these mutant molecules to send signals uninterruptedly, resulting in the uncontrolled proliferation of cells harboring mutations in this gene. This continuous division is what eventually leads to the formation of tumors in different parts of the body," explains Bustelo.
Precise methodology for precise treatment
A major impediment to the development of the study was that the low frequency of the genetic alterations made it impossible to perform causality studies using groups of cancer patients. To establish the real role of this genetic alteration in tumor processes, Xosé Bustelo's group decided to generate a genetically modified mouse in which this mutation could be induced at will by the researchers in postnatal stages. With this strategy, the aim was to mimic as closely as possible what happens in the earliest stages of human tumors: the appearance of a given genetic alteration in the healthy cells of an individual's adult organ and, from there, to see how these cells evolve in the long term.
"Subsequent analysis of tumor cells from each of these cancers allowed us to determine the changes that the RRAS2 mutation induced in the behavior of the cells that gave rise to the tumors and, as a result, to discover their therapeutic vulnerabilities," says Bustelo. "This research also allowed us to identify which targets and drugs would be the most suitable for eliminating tumors with mutations in this gene. Specifically, we have seen that the vast majority of RRAS2-induced tumors have as their main Achilles heel a molecule called mTORC1 for which drugs are already available," adds Laura Clavaín, a researcher at the Cancer Research Center (CIC-CSIC-USAL).
A target for Noonan syndrome
Outside the field of cancer, the results of the present study may also be useful for understanding the effects that mutations in the RRAS2 gene induce at the embryonic level to generate the congenital disease known as Noonan syndrome. This disease, which originates after the development of mutations in specific genes in the early stages of embryonic development, is associated with problems in the development of the head and the circulatory, muscular and nervous systems that ultimately severely affect the quality of life and long-term survival of the individuals who suffer from it. "Our work indicates that individuals affected with this syndrome are likely to have a high tendency to develop some tumor types as they progress into adulthood. It has also revealed what type of drugs could be of interest to correct some of the medical problems that individuals with this syndrome manifest," says Isabel Fernández-Pisonero, a researcher at the Institute of Biology and Molecular Genetics (IBGM-CSIC-UVA).
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in Spanish can be found here.
Original publication
Isabel Fernández-Pisonero, Laura Clavaín, Javier Robles-Valero, L. Francisco Lorenzo-Martín, Rubén Caloto, Blanca Nieto, Carmen García-Macías, Clara L. Oeste, Manuel Sánchez- Martín, Antonio Abad, Alejandro Hortal, Dolores Caballero, Marcos González, Mercedes Dosil, Balbino Alarcón, Xosé R. Bustelo; "A hotspot mutation targeting the R-RAS2 GTPase acts as a potent oncogenic driver in a wide spectrum of tumors."; Cell Reports.