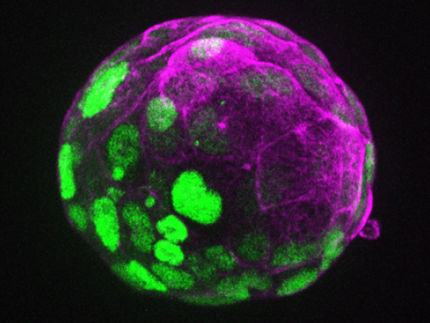
How the proper ratio of signaling molecules controls early embryonic development
Advertisement
During embryonic development, cells have to know their position and their future role in the organism, so that the tissues and organs of the body are created in the proper places. Two signaling molecules called Nodal and BMP are crucial for this. An interdisciplinary team led by Patrick Müller at the Friedrich Miescher Laboratory of the Max Planck Society in Tübingen has now found out how the distribution of these signaling molecules ultimately creates a code that assigns each cell its place and its function.
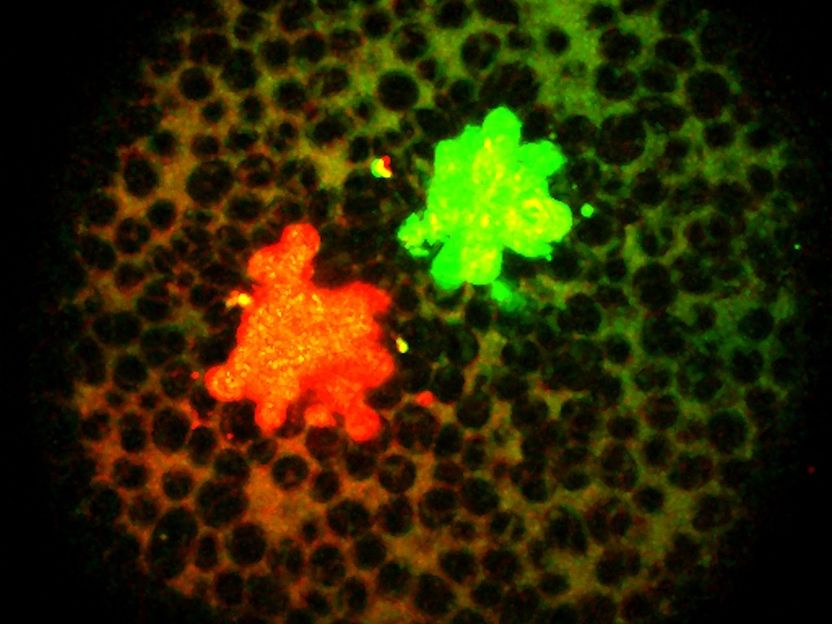
The signaling molecules labeled with fluorescent proteins.
Friedrich Miescher Laboratory, Tübingen/ Germany
This basic mechanism of early embryonic development may also help to guide tissue engineering approaches for regenerative medicine in the future.
It is like a well-choreographed dance: During early embryonic development, cells differentiate and move from an apparently chaotic clump to their final destination. In this way, patterns emerge from the cell clump, and the axes of the embryo develop along which the body parts emerge. Signaling molecules organize the choreography of this dance piece. They assign the immature cells their place in the embryo and ensure that they take on the intended role and function during their maturation process, so that for instance limbs or organs can form.
The choreographers, the key factors in early embryonic development, include the signaling molecules Nodal and BMP. Nodal determines where the interior of the embryo will be, and BMP determines where the belly (ventral) and the back (dorsal) will arise.
These two signaling molecules are also responsible for the formation of the head-to-tail body axis. Strikingly, when Nodal and BMP are artificially introduced into zebrafish embryos, these signaling molecules can even trigger the formation of a complete secondary axis – a Siamese twin.
The proper ratio between Nodal and BMP is decisive for the development of the axes. “A gradient along the axes is created from both molecules, and depending on how much Nodal or BMP is present, the fate of the embryonic cells in this area is determined,“ explains Gary Soh, first author of the publication. “If there is a lot of Nodal but little BMP, head structures are created; if the ratio is inverse and BMP is higher, a tail forms. However, so far it has been unclear how this gradient is created and how the cells of the embryo respond to this gradient.“
To address this question, Soh and colleagues labeled zebrafish Nodal and BMP with fluorescent proteins and tracked the formation of the resulting secondary axes. Since zebrafish embryos are transparent, the researchers were able to observe how both signaling molecules become distributed in the embryo. The surprising result: Nodal and BMP have very similar distributions.
So how are these distributions read out, such that the cells along the embryonic body axis assume different identies and perform different functions? This is where additional signaling molecules come into play: molecules of the so-called Smad family.
Nodal and BMP activate these molecules – but not equally, as the scientists found out. Using mathematical modeling and precise measurements, they showed that Nodal stimulates the activation of Smad2 relatively slowly, whereas BMP activates Smad5 very quickly.
As a consequence, different concentrations of the Smad molecules arise along the emerging axis. “We found that cells develop along different paths depending on the ratio between activated Smad2 and Smad5,” explains Soh. “These different ratios seem to create a kind of code that ultimately controls what cell type forms where“.
The scientists have revealed a fundamental mechanism of early embryonic development, which in the long term might also help to guide approaches for the differentiation of stem cells into specific tissues for regenerative medicine.
Original publication
Soh GH, Pomreinke AP, Müller P (2020); "Integration of Nodal and BMP signaling by mutual signaling effector antagonism"; Cell Reports, 31:107487