Toxin kills using dual mechanism
Advertisement
Photorhabdus bacteria live in worms that attack insects. The bacteria kill the insects, which then serve as a source of food for the worms as well as the bacteria. Studies in Australia and the USA have shown that these bacteria also cause inflammations of the skin and ulcers in humans. The substances responsible for this effect are bacterial toxins. A team of researchers led by Prof. Dr. Dr. Klaus Aktories from the Institute of Pharmacology and Toxicology of the University of Freiburg and BIOSS Centre for Biological Signalling Studies and Dr. Thomas Jank from the Institute of Pharmacology and Toxicology of the University of Freiburg has discovered the new toxin “PaTox” and elucidated its molecular mechanism.
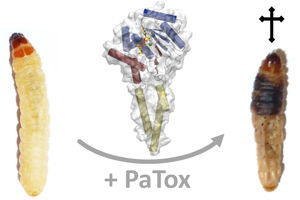
Effect of the bacterial toxin PaTox on the larvae of the greater wax moth (Galleria mellonella) and the crystalline structure of the effective part of the toxin
Thomas Jank - University of Freiburg
Also collaborating on the study were University of Freiburg research groups led by Prof. Dr. Carola Hunte, BIOSS Centre for Biological Signalling Studies and Institute of Biochemistry and Molecular Biology, and Prof. Dr. Bettina Warscheid, BIOSS Centre for Biological Signalling Studies and Institute of Biology II, as well as Prof. Dr. Hans Robert Kalbitzer from the Institute of Biophysics and Physical Biochemistry of the University of Regensburg. The team unraveled the structure of the toxic unit of PaTox. What is so special about the mechanism is that the toxin causes a sugar to be transferred to protein substances that serve as signaling switches in the host cell. The sugar residue attaches to the amino acid tyrosine. The scientists were able to observe this reaction for the first time ever. When the sugar attaches to the amino acid, the switch is set to “off.” This obstructs processes in the cell that regulate the cytoskeleton, causing the cell and insect to die. An unexpected finding for the researchers was that the cell’s signaling switch needed to be switched on for the toxin to take effect at all. Astonishingly, PaTox evidently turns on the switch itself. This requires a second mechanism of the bacterial toxin that causes the amino acid glutamine to be converted into glutamic acid, thus activating cellular signaling proteins.
The researchers have also found similar toxins in bacteria that cause diseases in fish, plants, and humans. The elucidation of the molecular mechanism of PaTox is crucial for the understanding of an entire family of toxins and lays the foundation for the development of therapeutic strategies for combating the bacteria that produce the toxin.
Original publication
Thomas Jank, Xenia Bogdanović, “A bacterial toxin that catalyzes tyrosine glycosylation of Rho and deamidation of Gq/Gi proteins”. Nature Structural and Molecular Biology. 2013.