FortéBio
Advance Lot Release and In-process Testing of Biologics in QC
FortéBio - A Sartorius Brand
Comprehensive technical and service support for Octet® users in GxP environments
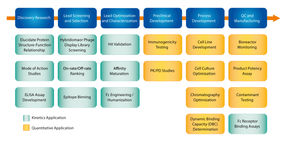
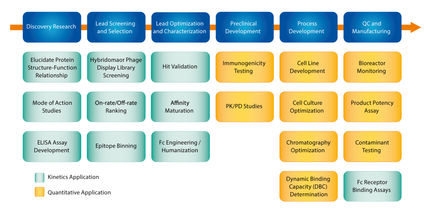
ForteBio’s Octet® systems utilize BLI technology to monitor biomolecular interactions in real time and label-free. They are an ideal replacement for ELISA and HPLC techniques for the quantification of antibodies and recombinant proteins and are especially suitable for product potency lot release assays. The plate-based and fluidic-free format also offers GMP users distinct advantages over comparative SPR based techniques. Octet systems provide higher throughput with the flexibility to run 2 to 96 samples simultaneously and better sample versatility, including the ability to analyze crude samples and more tolerance to diverse sample matrices. In addition, ease of use, low maintenance and high data precision speeds time to results throughout the drug development process.
This whitepaper describes the Octet platform for reliable and routine use under GxP regulations and particular in QC laboratories.
Advertisement