AutoTIL™ Microtumors
Patient-derived microtumors with preserved immune competence
Exclusive access to the only model worldwide with autologous TILs for true clinical relevance
More reliable prediction of therapeutic response through preserved immune competence
Broad use across solid tumors to accelerate and de-risk immunotherapy programs
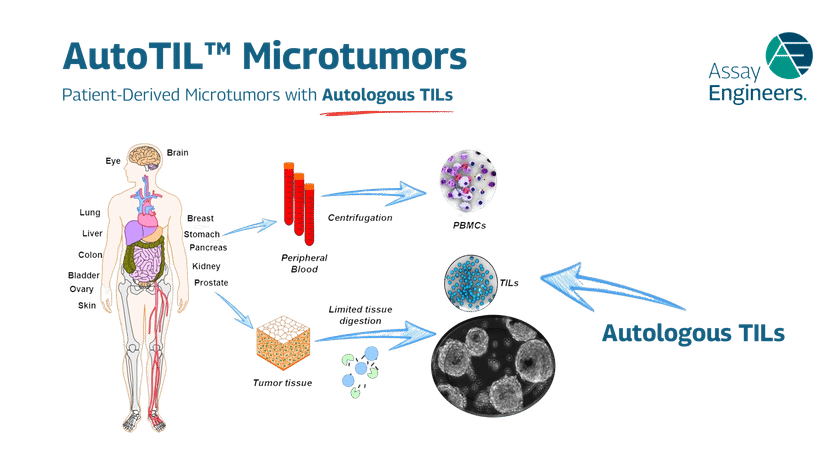
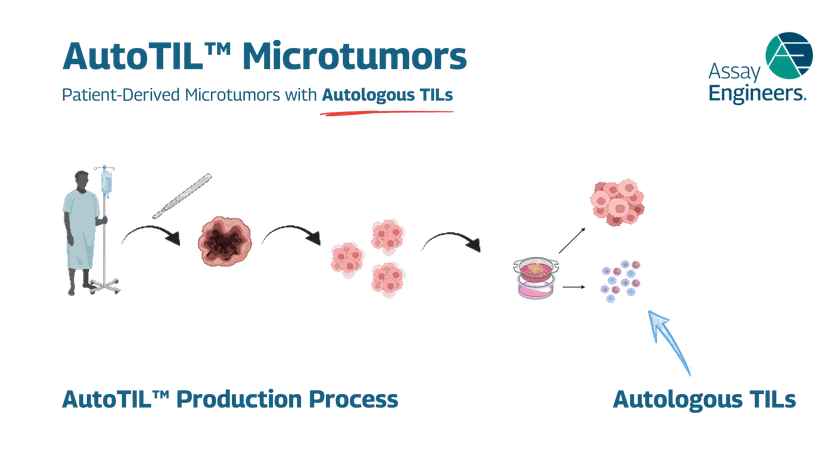



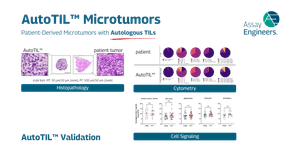

Clinically predictive models for advancing immuno-oncology drug development
AutoTIL microtumors represent a breakthrough in preclinical immuno-oncology research as the world’s first and only ex vivo model that integrates autologous tumor-infiltrating lymphocytes (TILs) with the patient’s own tumor tissue. By preserving the native immune competence, extracellular matrix, and cellular heterogeneity of the original tumor, AutoTIL microtumors overcome the limitations of conventional models that often fail to capture the complex interplay between malignant cells and the immune microenvironment. The result is a physiologically relevant system that is patient-specific, immune-competent, and non-alloreactive—offering researchers a uniquely predictive window into therapeutic response.
Unlike engineered co-culture systems or xenografts that rely on foreign immune components, AutoTIL microtumors maintain the authentic tumor–immune interactions of the donor patient. This provides an unprecedented opportunity to evaluate immune checkpoint inhibitors, cell therapies, bispecific antibodies, and novel immunomodulatory agents in a setting that mirrors real clinical biology. Because the system captures intrinsic mechanisms of resistance and sensitivity, researchers can identify biomarkers, refine drug candidates, and design combination strategies with greater confidence in their translational relevance.
The technology is designed for versatility and scalability. AutoTIL microtumors can be established from virtually any solid tumor type, including highly heterogeneous and difficult-to-model cancers. This broad applicability makes them a powerful platform across immuno-oncology research, spanning discovery, preclinical development, and translational studies. With a reliable workflow and consistent performance, research teams can generate reproducible results that inform decision-making at every stage of the drug development pipeline.
Access to AutoTIL microtumors is seamless, thanks to a straightforward fee-for-service model without licensing restrictions. This removes barriers to adoption and accelerates project initiation, enabling organizations to focus resources on advancing therapeutic innovation rather than navigating complex contractual frameworks. By delivering clinically predictive insights with unmatched fidelity, AutoTIL microtumors empower researchers to shorten development timelines, de-risk programs, and bring effective immunotherapies closer to patients in need.

1
Patient-derived microtumors with autologous TILs for clinically relevant immuno-oncology research

2
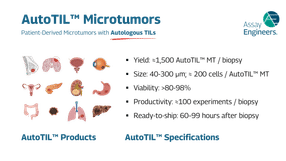
Production of AutoTILs – patient-derived microtumors with autologous TILs

3
Validation of AutoTILs – confirming clinically relevant patient-derived microtumors with autologous TILs

4
Product specifications – details on features and clinical relevance of AutoTILs

5
Download 3 new case studies now and gain insights from the latest research findings
Request information about AutoTIL™ Microtumors now

AutoTIL™ Microtumors
Patient-derived microtumors with preserved immune competence
Product classification AutoTIL™ Microtumors
Product categories
Applications
Manufacturers of similar products
Advertisement



