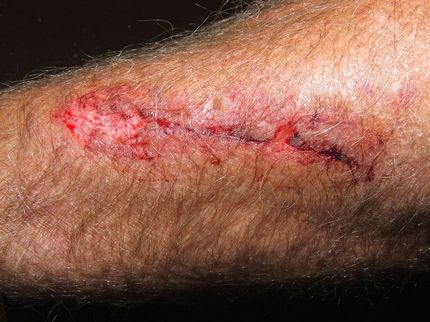
Heal-Or Announces Auspicious Results of Clinical Trials with HO-03-03 for Curing Chronic Wounds
Heal-Or, a biopharmaceutical company focused on the development of therapeutics for wound healing and various skin disorders announced auspicious results of phase I clinical trials with its leading product "HO-03-03" for curing chronic wounds, that are currently conducted in 3 medical centers in Israel. The company has recently begun the regulatory practice necessary for receiving FDA approval to conduct clinical trials in the US.
According to Heal-Or founder Dr. Tamar Tennenbaum: "In all of our preclinical studies, veterinary field trials as well as in our current phase I clinical trials, the 'HO/03/03' has shown to induce healing of non-healing diabetic ulcers and other types on chronic animal wounds as well as to significantly shorten time to heal and improve esthetics of chronic wounds". Dr. Tennenbaum says the encouraging results achieved in the pre-clinical stage enabled the company to pursue clinical trials in 3 Israeli medical centers, with substantially auspicious results already. The company is expected to receive an FDA approval to conduct clinical trials in the US at the beginning of 2007.
Heal-Or`s solution is based on a family of enzymes called Protein Kinase C (PKC), that instruct the skin to complete certain tasks at certain times during the wound healing process. Where one isoform of PKC may be involved in wound closure, another one may be responsible for keeping the wound uninfected or ensuring that the epidermal cells remain unscarred. By isolating particular isoforms and their respective inhibitors and activators, Heal-Or can moderate and stimulate certain steps of the wound healing process that are not working properly.
Most read news
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
National_Horse_Protection_League
Category:Vascular_surgery
Study finds breast and ovarian cancer may have similar origins
Evonik ends cooperation with Chr. Hansen
Category:Female_sexual_dysfunction_drugs

Frederick W. Alt and David G. Schatz to be awarded the 2023 Paul Ehrlich and Ludwig Darmstaedter Prize - Laureates raised knowledge of the development of the immune system to a new level
Chemotherapy timing is key to success - Nanoparticles that stagger delivery of 2 drugs knock out aggressive tumors in mice

Call for Entries: Eppendorf & Science Prize for Neurobiology 2017