Adipose-Derived Stem Cells Improved Left Ventricle Wall Thickness and Capillary Density in Pigs Following Myocardial Infarction
Advertisement
Cytori Therapeutics, Inc. announced that adipose-derived stem cells improved left ventricle wall thickness and capillary density following myocardial infarction in a randomized, placebo-controlled preclinical study. The study, conducted at Tulane University, involved administering fresh (uncultured) adipose-derived stem cells or placebo control intra-coronary to 17 pigs after severe myocardial infarctions. All animals received standard of care therapy. The only difference is that one group additionally received their own fresh adipose-derived stem cells. After an eight-week follow-up period, left ventricle wall thickness and capillary density were measured.
The heart wall thickness in the infarct area was greater in the adipose-derived stem cell group than in the placebo group (5.9mm vs. 3.6 mm), and a similar result was observed in the areas between undamaged and infarcted heart tissue (border regions of the heart - 11.2 mm vs. 8.6 mm). "This is significant as typically, heart attacks, even with standard of care, may result in a thinning heart wall in the area of damage which may lead to subsequent heart failure," said Alex Milstein, MD, VP of Clinical Development at Cytori Therapeutics. Capillary density, a measurement of blood supply, was also significantly increased in the border zones of the adipose-derived stem cell treated group compared to the control group. As expected, the healthy, undamaged myocardium showed no significant differences in the wall thickness or capillary density.
"We feel that these results help explain the positive impact of stem cell transplants for heart patients, demonstrating that they do, in fact, work to thicken the heart wall," said Eckhard Alt, M.D., of Tulane University. "We believe that stem cells from adipose tissue may be a valuable, novel alternative source for helping support cardiac regeneration after a heart attack."
These results are consistent with previously published functional improvements in multiple pre-clinical studies of adipose-derived stem cells and warrant further investigation of this treatment modality in human trials.
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

T-knife Completes €66 Million Series A Financing to Develop Next Generation T-Cell Therapies - T-knife’s proprietary humanized mouse platform (HuTCR) T-cell Receptors expected to provide superior affinity/specificity properties
Nanoparticles: Acidic alert
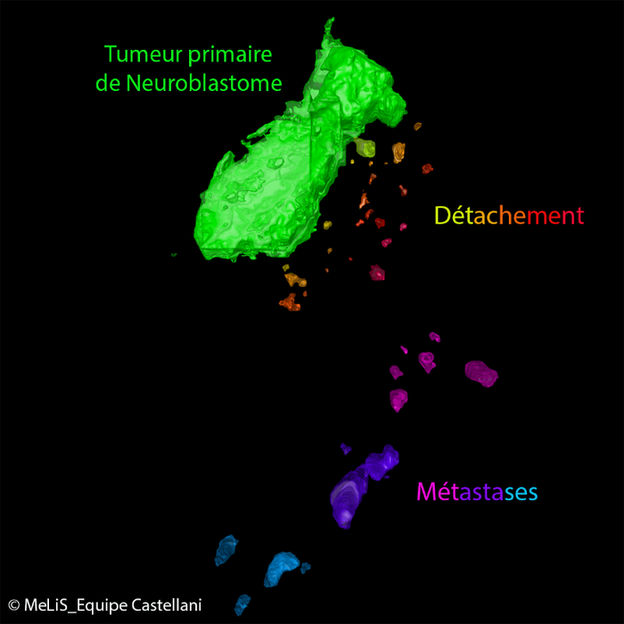
Healthy cells can impact tumour progression during embryonic development - Avian embryo model of neuroblastoma

LAUDA founds subsidiary in Italy
GenOway and Boehringer Ingelheim reinforce their collaboration
Advanced dosimetry phantoms improving radiotherapy verification
Detection of cancer from exhaled breath - Breath gas analysis for medical diagnostics
Dry eye syndrome: Neuroptis successfully completes the pre-clinical phase for ML7 - Neuroptis is looking to establish industrial and financial partnerships to commence human trials
TxCell receives Fast Track Designation from FDA for Ovasave - TxCell’s lead product designated for the treatment of moderate to severe Crohn’s disease