Genta completes marketing authorization application to EMEA for approval of Genasense in combination with chemotherapy
Advertisement
Genta Incorporated announced that the Company has completed a Marketing Authorization Application (MAA) to the European Medicines Agency (EMEA) that seeks approval of Genasense® (oblimersen concentrate for solution for injection) in combination with chemotherapy for the treatment of patients with advanced malignant melanoma.
Genta expects that the dossier will be formally reviewed for validation at a meeting of the Committee for Medicinal Products for Human Use (CHMP) that is scheduled for January 23-26, 2006. Assuming validation, the initial review of the MAA would commence thereafter, and Genta would anticipate receiving consolidated questions from the Agency approximately 120 days later. The centralized licensing procedure provides a single marketing authorization that is valid in all 25 member states of the European Community. Review of the application is coordinated by the EMEA, and Spain and France have been appointed as rapporteur and co-rapporteur countries, respectively.
Genasense, Genta's lead anticancer drug, is a novel targeted therapy that blocks the production of Bcl-2, a protein that appears to be a fundamental cause of resistance to cancer treatment. By knocking down Bcl-2 in cancer cells, Genasense may enhance the effectiveness of chemotherapy in patients with advanced melanoma.
Most read news
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Berkeley Lab researchers discover critical rotational motion in cells
Elusys and U.S. Army extend biodefense collaboration
Immune Defense Without Collateral Damage
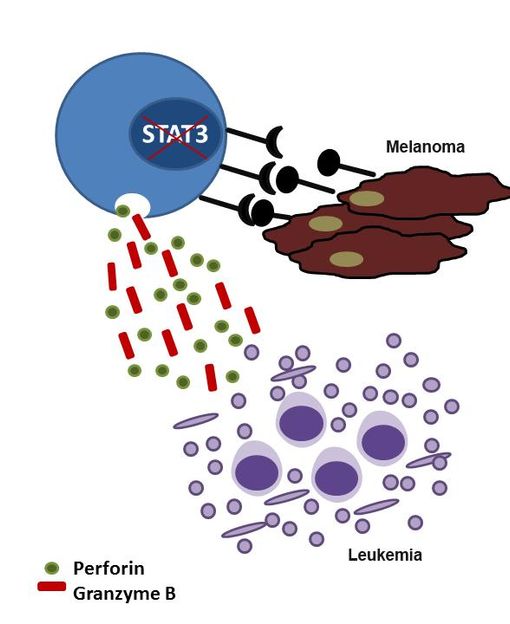
An unexpected bonus – blocking STAT3 could help cancer patients in two ways