Successful MHRA Inspection of Almac’s Clinical Services UK Facility
Almac announced the successful inspection of its Clinical Services operations located in Craigavon by the UK Medicines and Healthcare products Regulatory Agency (MHRA). The inspection, which occurred from 17th to 20th September 2012, was a routine inspection conducted by a MHRA inspector from the regulatory body who concluded that the site continues to be compliant with EU Good Manufacturing Practices, with no critical or major failures observed.
Almac has three MHRA approved clinical services facilities globally including our additional FDA assessed US facilities in Souderton, PA & Durham, NC.
Organizations
Other news from the department business & finance

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Cellular “power plants” control inflammation - Researchers discover how mitochondria not only produce energy but also influence inflammation

SOPAT GmbH and Malvern Panalytical announce a distribution agreement for US market
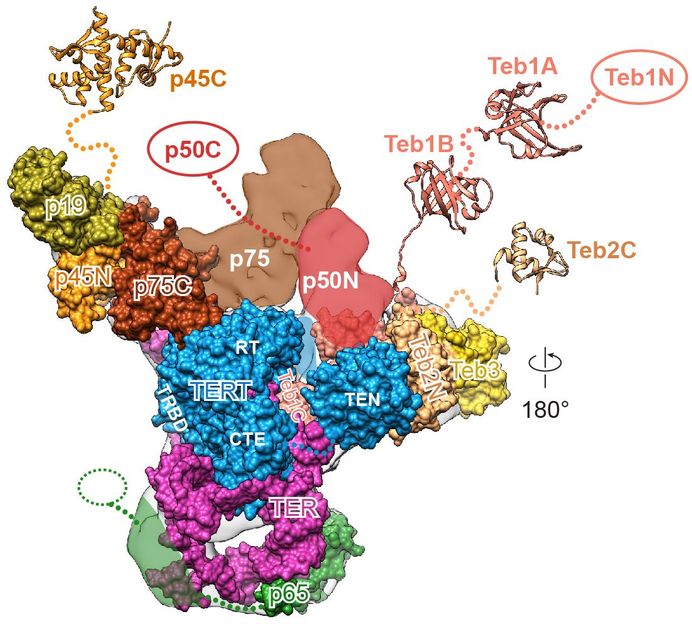
Scientists produce clearest-ever images of enzyme that plays key roles in aging, cancer
Movetis Announces Start of a Phase IIa trial for M0002 in Ascites

Scientists create a DNA test that identifies Lyme disease in horses - A test under development by a Rutgers professor could have applications for humans and dogs, too

FundaMental Pharma launches with EUR 10 million in Seed financing to advance a First-in-Class neuroprotectant - "These inhibitors have the potential to revolutionize therapies for currently untreatable neurodegenerative diseases"























































