Mini-laboratory delivers results in about 40 minutes at the point of care
Startup completes EU market approval for PCR rapid COVID-19 test
Advertisement
Spindiag GmbH, a fast-growing medical technology startup that developed a point-of-care testing system to detect bacterial and viral pathogens, announced that it has successfully completed the market entry assessment of its PCR rapid SARS-CoV-2 test. Spindiag’s Rhonda is now rolled out in phases in Germany, the EU, and other countries that accept the CE mark for in vitro diagnostics (CE-IVD). One of the first of these testing devices will be installed at Klinikum Stuttgart, which belongs to the largest hospitals in Germany. As dedicated Covid-19 care center of the metropolitan region, the Klinikum Stuttgart, where more than 100,000 tests on SARS-CoV-2 have already been conducted, plays an important role in managing the pandemic through diagnostics and patient care.
SpinDiag GmbH
The State Ministry of Economic Affairs, Labor and Housing provided EUR 6 million in funding at the beginning of the pandemic for the development of Rhonda.
PCR-based testing methods are considered the gold standard in infection diagnostics. They play a key role in Germany’s current national testing strategy, because they can detect the genetic information of SARS-CoV-2 and thus the virus itself directly and sensitively, thereby enabling accurate clinical decision making. Rhonda uses a highly reliable two-step PCR analysis method.
The mobile mini-laboratory only needs around 40 minutes for a test result that, with its robust performance data, is comparable to laboratory standards of PCR diagnostics. The comprehensive analytical performance data determines the limit of detection to be 7047copies/mL. A comparatively smaller performance evaluation using clinical samples detected one positive sample more than a selected laboratory reference system. Rhonda combines speed with the reliability provided by large-scale PCR laboratory tests – a development that will play an important role in future infection diagnostics.
Minister of Economic Affairs Dr. Nicole Hoffmeister-Kraut, said: “Until a vaccine is available, the most effective way to contain the virus is to identify infected people through rapid and broad testing. Together with the Hahn-Schickard Institute in Freiburg, Spindiag GmbH has succeeded in bringing a point-of-care rapid test to market in just a few months – this proves the enormous innovative strength and high level of competence of the companies and research institutions in Baden-Württemberg. Their innovative solutions are crucial in addressing global health threats such as the coronavirus pandemic. Time is an important factor in the fight against the pandemic. State funding enabled the coronavirus test to be developed in record time, demonstrating just how effective government grants can be.”
Prof. Dr. Jan Steffen Juergensen, Medical Director and Chairman of the Management Board of Klinikum Stuttgart, said: “Speed is often vital. More than 100,000 patients are admitted to Klinikum Stuttgart as emergency cases every year. Rapid diagnostics help medical professionals make the right decisions in acute situations. The new PCR rapid coronavirus test developed by Spindiag will be used from now on in the hospital’s emergency departments and combines the best of both worlds: the reliability of PCR-based testing and speed. Another advantage is that samples do not need to be sent to the laboratory for this point-of-care testing. I would like to thank the Eva Mayr-Stihl Foundation, which has continued to provide rapid and very generous support also during the pandemic and has enabled us to purchase several devices.“
Dr. Daniel Mark, CEO and co-founder of Spindiag GmbH, said: “Rapid tests are one of the most important measures to contain the coronavirus pandemic worldwide. Rhonda enables physicians and medical staff to use the PCR standard to test right after sample taking and without long waiting times due to logistics. We are very grateful to the State of Baden-Württemberg for the quick and unbureaucratic financial grant during the acute coronavirus crisis in April this year. We received the support along with the Hahn-Schickard Institute for Microanalytic Systems, the research facility from which we spun off in 2016. We are very pleased to have established the Rhonda testing system in the renowned Klinikum Stuttgart as one of the first hospitals in Germany.”
The long-term goal of the Freiburg startup is to develop a comprehensive and sustainable testing system for infection control. With its first product, the RhondaPCR rapid SARS-CoV-2 test, the company has brought a key technology to market that will provide diagnostic support for differentiated hygiene decisions by enabling numerous bacteria and viruses to be detected in a single analysis in the future – all in less than an hour.
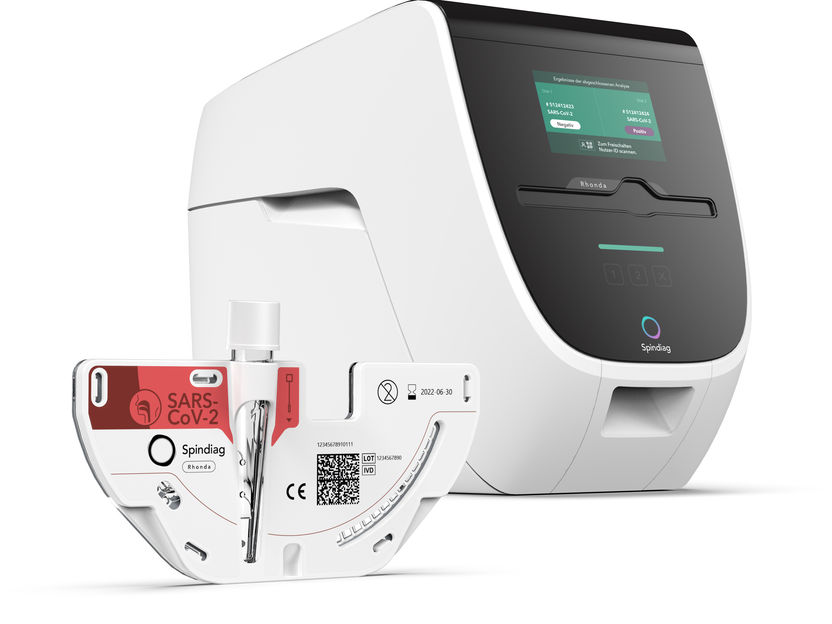
Spindiag Rhonda PCR rapid testing system
Spindiag’s fully automatic Rhonda rapid testing system consists of an analyzer with an insertable test cartridge. The Rhonda technology obviates the need for manual pipetting of samples into the test carrier, thereby reducing the risk of infection for users to a minimum. The testing system is easy to integrate into clinical routine. The test procedure itself is simple and user-friendly: swab samples can be inserted directly into the test cartridge, which contains all the reagents required for testing. Similar to a Compact Disc, the cartridge is then inserted into the analyzer and processed fully automatically. Another advantage is that two cartridges can be processed at the same time, thus enabling higher testing capacities. Test results can be read directly on the device and, if necessary, transferred digitally. Rhonda will be used in emergency departments, hospitals, fever clinics, physician offices specializing in COVID-19 testing, and nursing homes.
Other news from the department research and development
Most read news
More news from our other portals
Something is happening in the life science industry ...
This is what true pioneering spirit looks like: Plenty of innovative start-ups are bringing fresh ideas, lifeblood and entrepreneurial spirit to change tomorrow's world for the better. Immerse yourself in the world of these young companies and take the opportunity to get in touch with the founders.
See the theme worlds for related content
Topic World PCR
This groundbreaking and highly versatile molecular technique of PCR allows us to amplify tiny amounts of genetic material on a large scale and analyze them in detail. Whether in medical diagnostics, forensic DNA analysis or research into genetic diseases - PCR is an indispensable tool that gives us deep insights into the world of DNA. Immerse yourself in the fascinating world of the polymerase chain reaction (PCR)!

Topic World PCR
This groundbreaking and highly versatile molecular technique of PCR allows us to amplify tiny amounts of genetic material on a large scale and analyze them in detail. Whether in medical diagnostics, forensic DNA analysis or research into genetic diseases - PCR is an indispensable tool that gives us deep insights into the world of DNA. Immerse yourself in the fascinating world of the polymerase chain reaction (PCR)!


















































