News About Drug Delivery
Advertisement
nanocontainer for drugs can have their pitfalls: If they are too heavily loaded, they will only dissolve poorly. Why this happens is now reported by a Würzburg research group in "Angewandte Chemie".
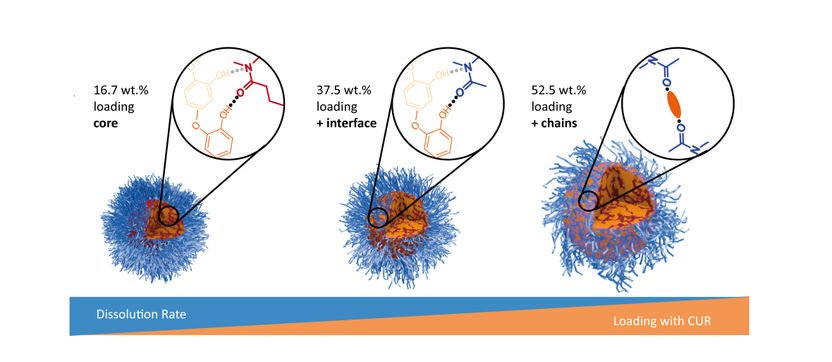
As the loading with curcumin (yellow) increases, the dissolution rate of the containers made of polymeric micelles (blue) decreases.
Ann-Christin Pöppler
Nanocapsules and other containers can transport drugs through a patient's body directly to the origin of the disease and release them there in a controlled manner. Such sophisticated systems are occasionally used in cancer therapy. Because they work very specifically, they have fewer side effects than drugs that are distributed throughout the entire organism.
This method is known in science as drug delivery. Chemistry professor Ann-Christin Pöppler from Julius-Maximilians-Universität (JMU) Würzburg in Bavaria, Germany, is convinced that this method still has great development potential. She analyzes the molecular capsules that enclose drugs like a container and transport them to the site of action: "My group wants to understand in as much detail as possible how the container molecules and the active substances arrange and what properties result from this," she says.
Polymeric micelles as research objects
The junior professor is mainly investigating polymeric micelles. These consist of many chains of molecules, which assemble into spherical structures. Such micelles are already on the market as drug containers. They are used in cancer therapies as well as in cosmetic products such as make-up remover lotions. When they come into contact with fat-soluble substances, they arrange themselves on their surface and at the end surround them like a coat of hair. This forms a container with a "water-loving" outer shell and a "fat-loving" core.
"Little is known about the molecular origin of the properties of these structures," says Pöppler. In the scientific journal Angewandte Chemie, the researcher and co-authors from JMU recently described an effect that is important for the design of future drug delivery systems: If increasing amounts of active ingredients are packed into the polymeric micelles, their dissolution suffers – the release of the active ingredients then becomes increasingly difficult.
Active ingredients glue the micelles together
The Würzburg research team found the reason for the decreasing solubility through a set of different experiments: As the container is loaded more and more, the active substances no longer settle exclusively in the core but also on the container surface. There they can almost glue the individual micelle hairs together. These molecular interactions reduce the solubility of the entire structure.
Next, the team hopes to find out whether the dissolution of the container can be improved by structural changes to the micelles. One of the goals of drug delivery is to ensure that a container absorbs as much active substance as possible and dissolves as well as possible in the body.
Polymer chemistry and pharmacy involved
Ann-Christin Pöppler cooperated with two other JMU groups in this work. The polymeric micelles were produced by Robert Luxenhofer, Professor of Polymer Functional Materials. The dissolution tests were carried out in the team of Professor Lorenz Meinel who heads the Chair of Pharmaceutical Technology and Biophysics.
The polymeric micelles used were compounds from the substance classes poly(2-oxazoline)s and poly(2-oxazine)s. Curcumin was used as model for an active substance because this ingredient of turmeric, a spice plant, is very easy to visualise spectroscopically. The structures of the containers loaded with different amounts of curcumin were determined by solid-state NMR spectroscopy and other analytical methods.
Original publication
"Loading-dependent Structural Model of Polymeric Micelles Encapsulating Curcumin by Solid-State NMR Spectroscopy"; Ann-Christin Pöppler, Michael M. Lübtow, Jonas Schlauersbach, Johannes Wiest, Lorenz Meinel, Robert Luxenhofer; Angewandte Chemie; 2019