MolYsis
MolYsis: Selective Enrichment and Isolation of Bacterial and Fungal DNA from Host Material


Removal of Host DNA for Sensitive and Specific Molecular Analysis of Bacterial and Fungal Targets
Host DNA negatively influences the sensitivity and specificity of molecular analysis of microorganisms in human and animal samples.
The groundbreaking MolYsis technology provides a solution for the removal of non-target host DNA and enrichment of intact microorganisms. Applications comprise next generation sequencing, amplicon cloning, real-time PCR, Sanger sequencing and DNA array analysis of microorganisms.
Protocols are available that allow the MolYsis pre-treatment of 0.2-10ml samples followed by microbial DNA extraction and purification. The MolYsis Complete kits include the entire process of MolYsis pre-treatment and microbial DNA purification.
The MolYsis Basic kits include the removal of host DNA and enrichment of microbial DNA and can be combined with any other commercial DNA purification kit. A great variety of liquid samples can be processed, among them whole blood, CSF, BAL, synovial fluid, pleural fluid, urine and others.
Tissue biopsies and other materials as well as liquid samples can be extracted using the Ultra-Deep Microbiome Prep kits.
A walk-away automated version of the MolYsis technology, MolYsis-SelectNA plus, is available as well. All kit components are guaranteed free of contaminating microbial DNA.
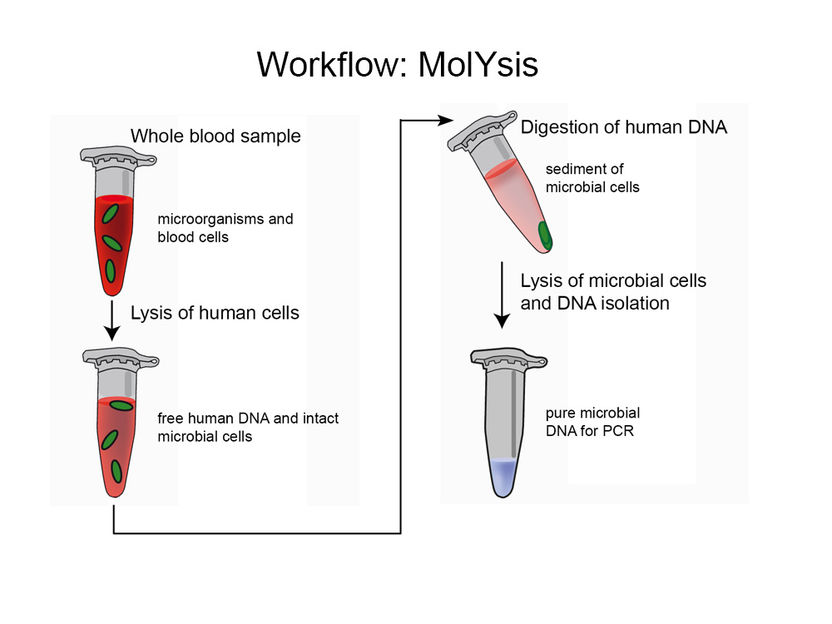
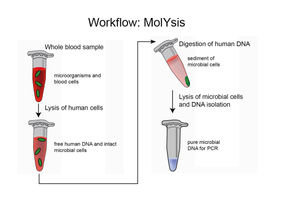
The Process of Selective Isolation of Bacterial and Fungal DNA
The MolYsis technology includes a process by which non-target DNA is liberated from the host cells by a selective lysis of human or animal cells. In the following extraction steps, the host DNA is digested by a DNase treatment, a reagent lyses a broad variety of Gram-positive and Gram-negative bacteria and fungi. Further, PCR inhibitors are removed, finally providing pure, enriched microbial DNA.
MolYsis has been validated with more than 200 genera of bacteria (86 Gram-positives, 120 Gram-negatives) and 65 genera of fungi from the clinical routine.
Key Benefits:
- Removal of non-target, human or animal DNA
- Selective enrichment of bacteria and fungi
- Removal of PCR inhibitors
- Validated for a broad-range lysis of bacteria and fungi
- Guaranteed DNA-free reagents
- Sample volume 0.2-10 ml
- Protocols for tissue samples available
- Automation available
Optimal detection of microbial DNA in clinical specimens is achieved by the combination of MolYsis extraction with Molzym‘s highly active, DNA-free MolTaq 16S/18S polymerase or with the ready-to-use DNA-free Mastermix products.

1

2

3
Request information about MolYsis now

DNA extraction kits: MolYsis
MolYsis: Selective Enrichment and Isolation of Bacterial and Fungal DNA from Host Material
Product classification MolYsis
Product categories
Applications
Manufacturers of similar products
Advertisement
Find more DNA extraction kits and related products
Find MolYsis and related products in the theme worlds
Topic World PCR
This groundbreaking and highly versatile molecular technique of PCR allows us to amplify tiny amounts of genetic material on a large scale and analyze them in detail. Whether in medical diagnostics, forensic DNA analysis or research into genetic diseases - PCR is an indispensable tool that gives us deep insights into the world of DNA. Immerse yourself in the fascinating world of the polymerase chain reaction (PCR)!

Topic World PCR
This groundbreaking and highly versatile molecular technique of PCR allows us to amplify tiny amounts of genetic material on a large scale and analyze them in detail. Whether in medical diagnostics, forensic DNA analysis or research into genetic diseases - PCR is an indispensable tool that gives us deep insights into the world of DNA. Immerse yourself in the fascinating world of the polymerase chain reaction (PCR)!