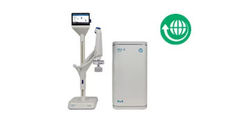
Sartorius
Sartocheck® 5 Plus
Filter integrity tests with automatic quality risk management

The Sartocheck® 5 Plus represents the ideal intersection point of today’s most relevant industry requirements for filter integrity testing within demanding GMP environments. A combination of a unique approach to Quality Risk Management (QRM) as well as optimal data integrity, intuitive usability, and minimized risk factors for Health, Safety, and Environment (HSE) set a new standard for filter integrity test devices.
Surpass the Requirements of QRM
The regulatory focus on QRM (cf. ICHQ9 and the new Annex 1 written by EMA in cooperation with the US-FDA, WHO, and PICs) also applies to filter integrity testing, as a fundamental element of sterility assurance.
The Sartocheck® 5 Plus Filter Tester uses program-specific parameters allowing the automatic identification of testing anomalies before or during the test. This prevents time-consuming, costly variations, potential drug recalls,and 483 warning letters.
Experience the Comfort of Intuitive Usability
An optimal user experience speeds up process workflows due to intuitive guidance and ease of use. The high-quality touchscreen of the Sartocheck® 5 Plus Filter Tester provides a unique viewing angle, an intuitive user interface, a logical menu structure, and simple data entry options. This allows straightforward programming of tests and QRM enhancement features, as well as error-free operationin GMP production environments.
Reach the Ultimate Level of Data Integrity
Filter integrity test values are part of the batch protocol and are used to justify the drug release. Long-term reliable data is crucial to avoid quality deviations and potential 483
warning letters.
The integrity and security of filter integrity test data must not be seen only as an IT problem, but also as a potential global business risk. Low standards of data integrity and security may not only jeopardize the activities of the drug manufacturing company, but more critically, endanger the health of patients.
Discover the Simplicity of HSE
Integrity testing often involves the use of chemicals and hazardous materials, e.g., alcohol. The Sartocheck® 5 Plus is certified for use in explosion-hazardous areas (ATEX) and is compatible with all current cleaning agents and VHP. This ensures maximum safety for operators and manufacturing facilities
Product classification Sartocheck® 5 Plus
Product categories
Applications
Manufacturers of similar products
Advertisement