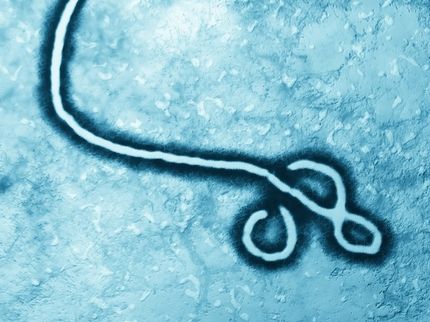
Crucell and NIH VRC Announce Start of Ebola Vaccine Clinical Trial
Advertisement
Crucell N.V. announced that the Ebola vaccine it is developing in partnership with the Vaccine Research Center (VRC) of the National Institute of Allergy and infectious diseases (NIAID), part of the U.S. National Institutes of Health (NIH), has commenced its Phase I clinical study. The randomized, double-blind, placebo-controlled study in 48 healthy volunteers will test the single-shot vaccination in a dose-escalation trial.
The start of the trial follows the successful completion of the Investigational New Drug (IND) application process required by the Food and Drug Administration (FDA) in the US. In preclinical studies, a single shot of the PER.C6®-based vaccine protected monkeys completely against a lethal Ebola challenge. The Phase I study will be carried out by the VRC at the NIH Clinical Center in Bethesda, Maryland. The main factors under examination are the vaccine's safety, tolerability and immunogenicity.
Most read news
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.