Interaction between two leukemia drugs explained
Advertisement
Currently no treatment option is available for five percent of patients suffering from chronic myelogenous leukemia, since they have developed resistance to conventional medications. Prof. Stephan Grzesiek’s group at the Biozentrum of the University of Basel, in collaboration with Dr. Wolfgang Jahnke and colleagues from Novartis, has investigated the combined action of two different compounds against this form of leukemia. They have been able to explain at the atomic level, how both substances alter the structure of an enzyme and how their combination potentially can overcome drug resistance.
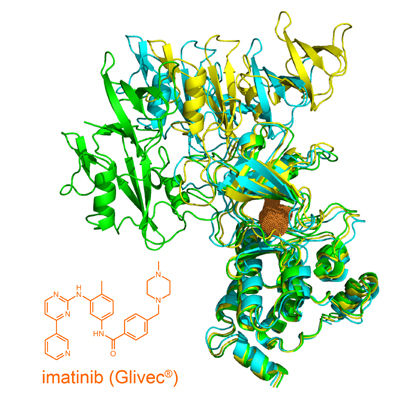
Structure of the open tyrosin kinase-imatinib complex.
University of Basel
Chronic myeloid leukemia (CML) is a form of blood cancer based on a genetic disorder that leads to the overproduction of white blood cells. Ninety-five percent of affected patients can be treated successfully with the Novartis drug imatinib, also known as Gleevec®. Imatinib is an inhibitor that blocks the ATP-binding site of the tyrosine kinase Abl in affected blood cells, thereby suppressing their overactivity. Consequently, the pathological overproduction of leucocytes is stopped and the blood count normalizes.
Five percent of all patients are not cured by imatinib
However, in five percent of CML patients, typically in an advanced stage of the disease, imatinib and similar ATP-binding site inhibitors are not effective. This resistance against treatment is caused by a mutation at the ATP-binding site, which prevents the inhibitors from inactivating the enzyme. Currently, new treatments are being developed to help such resistant patients. One approach is based on the combination of ATP-binding site inhibitors with so-called allosteric inhibitors, which bind to a different location.
Why the drug combination works in resistant CML
Why such a combination of the two inhibitor types works in an animal model has now been explained by Prof. Stephan Grzesiek‘s team at the Biozentrum of the University of Basel and Dr. Wolfgang Jahnke from Novartis, by a structural analysis using nuclear magnetic resonance spectroscopy (NMR). Under physiological conditions, the tyrosine kinase Abl is found in two different spatial structures - an open and a closed state - which exist in a delicate equilibrium. The researchers have shown that the binding of imatinib unexpectedly shifts this equilibrium to the open state. Although the enzyme itself is inhibited in this state, it can be more easily re-activated through other tyrosine kinases. The allosteric inhibitor GNF-5, however, stabilizes the closed, inactivated state, and even recloses the imatinib-induced open state.
“Thus the inhibitory potentials of both drugs add together to suppress the kinase activity. Our structural analysis enables us to understand why GNF-5 contributes to overcome imatinib resistance,” explains Lukasz Skora, a former postdoc from Stephan Grzesiek’s lab. These results provide a detailed insight into how Abl kinase behaves under the influence of inhibitors, giving hope for a successful combination therapy.
Original publication
Lukasz Skora, Jürgen Mestan, Doriano Fabbro, Wolfgang Jahnke, and Stephan Grzesiek. NMR reveals the allosteric opening and closing of Abelson kinase by ATP-site and myristoyl pocket inhibitors. Proceedings of the National Academy of Sciences PNAS, Published online 4 November 2013.