Cancer cells on the wrong path
How adaptations can make cancer treatment more difficult
Advertisement
Each tumor consists of multiple cell types, all of which have distinct characteristics. In addition to determining the course of the disease, these differences between individual cancer cells control the effectiveness of targeted therapies. A team of researchers from Charité – Universitätsmedizin Berlin and the German Cancer Consortium (DKTK) has been able to trace the developmental trajectories of colorectal cancer cells. In addition to observing how individual cells respond to cancer therapies, the researchers were also able to see the way in which cancer cells sometimes develop resistance following treatment. Writing in EMBO Molecular Medicine, the researchers suggest that this knowledge can be used to identify where current treatment approaches fall short and how these shortcomings might be improved.
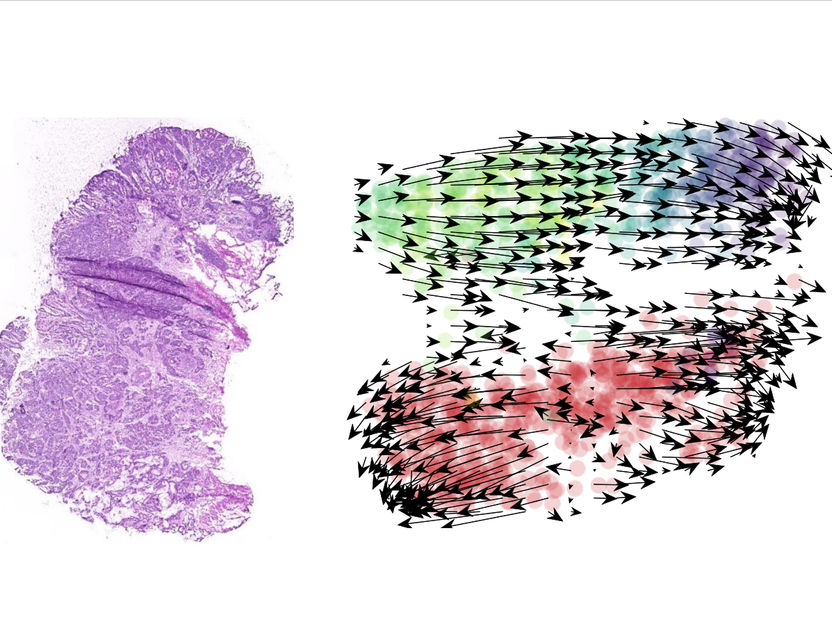
From cancer tissues to the developmental trajectories of single cells. A tissue section (left) will provide important information on a patient’s cancer. A bioinformatics analysis of single cell data (right) shows that, in the absence of treatment, cancer cells in organoid cultures will follow a uniform trajectory from the early to the late stages in the life cycle (arrows, from green to blue). With treatment (red), cancer cells will develop along multidirectional trajectories.
© Charité | Markus Morkel
A technique known as single cell sequencing enables researchers to study gene expression – the activity of individual genes – across thousands of cells at the same time. Tumors consists of subpopulations of cells which, in addition to having different characteristics, continually adapt to changes in their microenvironment. Using single cell sequencing, a team of researchers led by PD Dr. Markus Morkel and Prof. Dr. Nils Blüthgen from Charité’s Institute of Pathology studied the inherent heterogeneity of tumor tissues in order to gain a clearer picture of the colorectal cancer development process.
The researchers started by comparing colorectal cancer cells with cells found in healthy gut tissue. By collecting data on a total of more than 100,000 individual cells, they were able to define cell characteristics which were universal across individual patients. Conventional sequencing methods only provide a picture of gene activity at a specific moment in time. In order to recreate and observe the dynamic changes taking place in tissues at the cellular level, the researchers had to create three-dimensional cultures of colorectal cancer cells. “Using these ‘organoids’ as they are called, we were able to trace the cells’ developmental trajectory,” explains PD Dr. Morkel, adding: “Thanks to a clever laboratory technique, we were able to label the cells’ RNA at a specific point in time. In addition to determining the current status of activity for each individual cell, this technique also provided us with a picture of gene expression from a few hours earlier.” The research team then examined the way in which the cancer cells within these organoids adapted to clinically important therapies with targeted inhibitors. Not all of the cancer cells responded in the same way. While some cells were extinguished by this treatment, others took what might be best described as ‘a wrong turn’. Deviating from their normal developmental trajectories, they entered a new state which rendered them resistant to the recently administered treatments.
“These types of single cell experiments with cancer tissues represent a huge logistical and technical challenge. They involve collaborations between specialists across multiple facilities – ranging from surgical specialists to database experts,” explains PD Dr. Morkel, who also serves as the main point of contact at the BIH at Charité’s Bioportal Single Cells, a core facility which aims to enable the fast and efficient incorporation of single cell technologies in near-patient translational research. One obvious challenge is addressed by Prof. Blüthgen, who is also a researcher at the Integrative Research Institute (IRI) for the Life Sciences at the Humboldt-Universität zu Berlin (HU): “Measuring the activity of thousands of genes in hundreds of thousands of cells produces very large volumes of data,” he explains, adding: “It is mainly due to advances in the field of machine learning that we are now in a position to analyze these data in an efficient way. This enables us to gain a better understanding of the essential cellular processes and then use this for the benefit of patients.” Single cell experiments, machine learning and patient-specific cell culture models are set to play a key role in the research and development of new cancer treatment options at Charité.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Cell Analysis
Cell analyse advanced method allows us to explore and understand cells in their many facets. From single cell analysis to flow cytometry and imaging technology, cell analysis provides us with valuable insights into the structure, function and interaction of cells. Whether in medicine, biological research or pharmacology, cell analysis is revolutionizing our understanding of disease, development and treatment options.
Topic World Cell Analysis
Cell analyse advanced method allows us to explore and understand cells in their many facets. From single cell analysis to flow cytometry and imaging technology, cell analysis provides us with valuable insights into the structure, function and interaction of cells. Whether in medicine, biological research or pharmacology, cell analysis is revolutionizing our understanding of disease, development and treatment options.
















































