Chimera Biotec
Immunoassay support for clinical PK assessment where ELISA fails
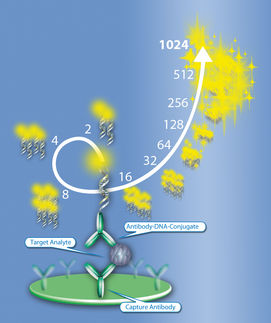
Imperacer®: Advantages in high sensitivity and dilution tolerance for challenging PK support
ELISA still is the gold-standard in immunoassays, providing sufficient sensitivity for the majority of pharmacokinetic (PK) studies. Modern biotherapeutic drugs however, may require bioanalytical sample testing with significantly increased assay performance over classical ELISA. Depending on the drug´s specifications and mechanism of action PK profiling may demand extreme immunoassay sensitivities or related features.
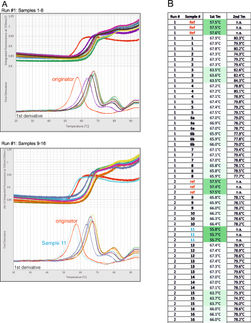
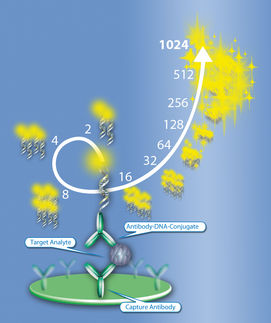
Here we summarize three case studies where the exponential signal amplification of the Imperacer® technology was used to fulfill the requirements of clinical PK assessment of such novel biologics.
Bioanalytical PK support for the development of highly efficacious peptide drugs in diabetes treatment and chronic pain relief / autoimmune disorders clearly demanded highest immunoassay sensitivities in low pg/ml range to assess full PK profiles. In contrast, PK assessment of a fusion-drug in replacement therapy was limited by strong matrix interaction in ELISA by high levels of interfering endogenous counterpart. Here, Imperacer® intrinsic sensitivity was used to optimize the assay towards high dilution tolerance to minimize effects from the endogenous compound, still maintaining needed sensitivity towards the drug.
Wherever ELISA supports reaches technical limitations, PCR driven Imperacer® sensitivities provide powerful alternative, with the possibility for fit-for-purpose validation of the method according to regulatory guidance.
Advertisement
White Paper classification
White papers on related topics
Products on related topics
Manufacturers of similar products
See the theme worlds for related content
Topic World PCR
This groundbreaking and highly versatile molecular technique of PCR allows us to amplify tiny amounts of genetic material on a large scale and analyze them in detail. Whether in medical diagnostics, forensic DNA analysis or research into genetic diseases - PCR is an indispensable tool that gives us deep insights into the world of DNA. Immerse yourself in the fascinating world of the polymerase chain reaction (PCR)!

Topic World PCR
This groundbreaking and highly versatile molecular technique of PCR allows us to amplify tiny amounts of genetic material on a large scale and analyze them in detail. Whether in medical diagnostics, forensic DNA analysis or research into genetic diseases - PCR is an indispensable tool that gives us deep insights into the world of DNA. Immerse yourself in the fascinating world of the polymerase chain reaction (PCR)!