Aҫaí berry extracts fight malaria in mice
Despite humanity's best efforts to eradicate malaria, the disease struck more than 200 million people in 2017, according to the World Health Organization. Worse yet, the parasite that causes malaria is developing resistance to many antimalarial drugs, including the mainstay, chloroquine. Researchers are actively searching for new treatments, and now, a group reporting in ACS Omega have found that aҫaí berry extracts can reduce parasites in the blood and prolong the survival of infected mice.
Aҫaí is native to Brazil, where some traditional healers use the berries to treat malaria symptoms. In recent years, the high antioxidant content of the grape-like fruit has boosted its popularity outside of Brazil and has caused some to consider it a "superfood." This antioxidant activity arises mainly from polyphenols -- compounds that have been linked to health benefits such as weight loss, cardiovascular disease prevention and decreased cancer risk. Susanne Mertens-Talcott, Fabio Costa and colleagues wanted to determine if aҫaí extracts could treat malaria in mice, and if so, whether polyphenols in the berries were responsible for the therapeutic effect.
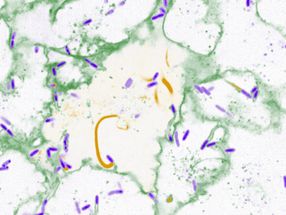
The team extracted polyphenols from aҫaí berries and then treated malaria parasite cultures growing in a Petri dish with the extracts. They found that a class of polyphenols called nonanthocyanin phenolics inhibited the growth of both chloroquine-resistant and -sensitive parasites. Then, the researchers orally administered aҫaí polyphenols to malaria-infected mice. The treatment reduced the parasitic load in the mice's blood by 89.4% compared with untreated mice. All of the mice given polyphenols survived for more than 15 days, whereas none of the untreated mice lived. The aҫaí extracts appeared to interfere with the parasites' protein homeostasis, or the balance between protein production and degradation, the researchers say.
Original publication
Other news from the department science

Get the life science industry in your inbox
From now on, don't miss a thing: Our newsletter for biotechnology, pharma and life sciences brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.