Photoreactive compound allows protein synthesis control with light
Okayama University researchers control the timing and location of protein synthesis using a photoresponsive compound that is an inactive key molecule until it is activated by brief irradiation.

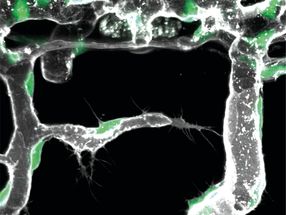
Photoinduced DsRed synthesis in a mammalian cell. DsRed was synthesized only within the irradiated cell
Okayama University
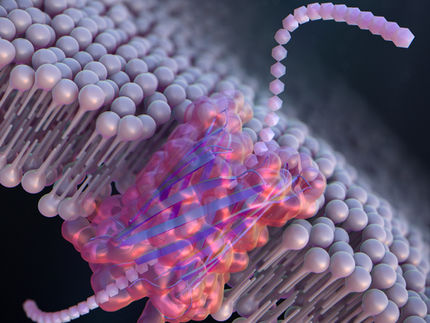
The production of proteins at distinct times and locations regulates cell functions, such as cell development and differentiation into specific cell types. The ability to exert spatiotemporal control over protein synthesis would help investigate these processes. Now Takashi Ohtsuki and colleagues in the Department of Medical Bioengineering at Okayama University, Japan, have shown that they can prevent protein synthesis reactions from taking place using a photoresponsive molecular cage (see Figure). Brief exposure to light releases key protein synthesis molecules from the cage without damaging them, so that protein synthesis takes place at the time and location of irradiation.
In their study, the researchers used an aminoacyl-transfer RNA (aa-tRNA), a molecule that helps decode messenger RNA so that protein synthesis can occur. Previous work had successfully inhibited protein synthesis by protecting aa-tRNA with a molecular cage of nitroveratryloxycarbonyl group. However the 30 minutes of light exposure required to release the aa-tRNA from the cage caused damage that then inhibited the protein’s function.
Instead Ohtsuki’s team used (7-diethylaminocoumarin-4-yl)methoxycarbonyl (DEACM) group. While the caged aa-tRNA was stable for at least 4 hours, irradiating for just 20 seconds released the aa-tRNA. The researchers demonstrated light-controlled activity of the caged aa-tRNA in vitro, in a gel, in liposomes and in mammalian cells.
“This method of spatiotemporally photocontrolling translation offers a promising approach for investigating the relationship between local translation and biological functions,” conclude the researchers in their report of the work. As well as a tool for protein synthesis investigations the team suggest it could also be used to insert artificial amino acids into proteins in a controlled manner for further studies.
Original publication
Most read news
Original publication
Takashi Ohtsuki, Shigeto Kanzaki, Sae Nishimura, Yoshio Kunihiro, Masahiko Sisido & Kazunori Watanabe; "Phototriggered protein syntheses by using (7-diethylaminocoumarin-4-yl)methoxycarbonyl-caged aminoacyl tRNAs"; Nature Comm.; 2016
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
From now on, don't miss a thing: Our newsletter for biotechnology, pharma and life sciences brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.